Reflections on Angelika Amon’s life and legacy by Kim Nasmyth
Angelika Amon was one of the first students to start at the brand-new IMP when she joined the lab of Kim Nasmyth, one of the institute's first Senior Group Leaders, in 1987. As the budding institute started to unfold, so did Angelika's career. In his personal tribute to Angelika’s legacy, Kim reflects on the early stages of her career and the ties between the two and the IMP.

It is very hard for me to grasp that Angelika is no longer with us. She was such a strong presence that her loss will leave a gaping hole in all our lives. As her supervisor during both her undergraduate diploma and her doctorate, I had the great privilege to work with her at the birth of her scientific career. Given her natural talent, it is questionable how much I influenced her. However, in the light of her subsequent career, I certainly did not inflict any lasting intellectual damage. What is clear is that her work had a huge influence on my career as I will later try to explain.
Angelika turned up within days of my move to the IMP in the winter of 1987/88. The labs had been created by converting an old electronics factory, which was still surrounded by grey concrete ruins. If I may have had doubts as to why I had come here, so too must have Angelika. Dr.-Bohr-Gasse was situated in one of the greyer parts of the third district, a short dark alley off the Rennweg. To get there from the University, you had to take the 38 tram into town and then the 71 along the Rennweg, an infamous street referred to as the gateway to the Balkans by Metternich. In other words, you did not just turn up. You had to head off to Eastern Europe, which at that time was still fenced off behind the iron curtain. It was all a far cry from smart thirteenth district near the Schönbrunn palace where Angelika had grown up.

The lab was empty and still bereft of equipment. Despite this and despite its location far from other students and faculty, Angelika was insistent that she wished to work with me for her Diplomarbeit. As it happens, the lack of equipment was no great hindrance as she embarked on a genetic screen that required little more than petri dishes, toothpicks, and a dissecting microscope I had smuggled in from Cambridge. I was still working full time in the lab and we occupied neighbouring benches for the next four years. I was the main beneficiary of this arrangement as I insisted that we conduct part of our discussions in German. Though this may not have helped the project, and might indeed have crippled it intellectually from time to time, it was an invaluable learning experience for me.
It soon became clear that we shared more than just a bench in common. Whether in German or English, Angelika always spoke her mind and was happy to mete out verbal or intellectual punishment when others deserved it. Crucially, she never had a problem when this was reciprocated. These were aspects of a culture that I had grown used to at the LMB in Cambridge and ironically had part of its roots in Viennese exiles such as Max Perutz. Though ostensibly this was a teacher/pupil situation, it never felt like that. It was a collaboration where she did all the heavy lifting in the lab and I merely shared in the discussion. It was also apparent that Angelika tended to formulate her views and hypotheses in response to new facts very rapidly and long before she was subsequently able to articulate them. As John Maynard Keynes put it, “when the facts change, I change my mind”. She had the energy and experimental virtuosity to produce lots of new facts and in response changed her mind very rapidly, almost spontaneously. This was an indication of a prodigious intelligence lurking within her subconscious. There is little doubt that all intellectual heavy lifting is indeed of this nature and that language per se contributes rather little to this process, serving merely to persuade others of the righteousness of your cause. Though true for us all, this was especially clear in Angelika. This is why I once told her that she tended to think not with her brain but with her hands. Though it took longer to develop, Angelika subsequently became equally adept in articulating her ideas. This was just as well, because she was at heart an extrovert who needed to communicate, which she usually did at full steam and with little restraint. Though I never fully witnessed her in action in her lab at MIT, I can fully imagine how her relentless subconscious imagination and desire to communicate combined to make her such a formidable and inspiring leader.
Angelika and the early years of the IMP
I have several other vivid memories of Angelika in the early days of the IMP: her insistence that we all at least attempt the Wiener Walzer at Faschingsparties; her beautiful wedding to Johannes in Otto Wagner’s masterpiece, the Kirche am Steinhof, which took place in depths of winter and was so cold her marriage vows swirled into vapour trails; and her visit to our mountain chalet in Carinthia, when she disappeared into the woods for an hour or so and reappeared clutching several magnificent specimens of Steinpilze (cep), which was our first introduction to the joys of hunting for wild mushrooms.

Unfortunately, or in hindsight perhaps not, her efforts during her Diplomarbeit did not yield much edible fruit, primarily because the premise behind her genetic screen was fundamentally flawed. Angelika wanted to study how cell division was controlled. A wonderful example, beautifully characterized by Lee Hartwell and his colleagues, was the arrest of haploid yeast cells in the G1 phase of the cell cycle in response to pheromones secreted by cells of the opposite mating type. This mutual cell cycle arrest was an essential prelude to conjugation and thereby the formation of diploid cells. Lee rightly assumed that there must be genes specifically concerned with the mechanism by which pheromones prevent yeast cells from initiating a new cell cycle. Identifying such genes would help reveal how the Cdc28 (Cdk1) protein kinase orchestrated cell cycle initiation, known as Start. He reasoned that mutation of such a gene would cause cells to be unresponsive to pheromone and therefore sterile. He also rightly recognized that many if not most mutants unresponsive to pheromone would prove to be defective in the pathway by which pheromones bind to their receptors and transduce a signal within the cell. In other words, they would be revealing about pheromone signal transduction but not per se about the specific branch of the pathway concerned with inactivating Cdc28. Having previously shown that yeast cells with an inactive Cdc28 protein were capable of conjugating, Lee reasoned that the sterility of mutants defective specifically in inactivating Cdc28 in response to pheromone should be rescued by inactivating Cdc28 itself using a temperature sensitive allele. As it happens, none of his unresponsive ste mutants possessed this property and instead all of the seven STE complementation groups (genes) identified by his screen were concerned with the process of signal transduction.
Master thesis and an initial setback
Angelika and I guessed, wrongly as it turns out, that the reason why Lee had failed to identify cell cycle arrest genes was that they were also necessary for cell cycle progression and therefore were essential genes. In other words, the lethality caused by their inactivation would preclude continued proliferation in the presence of pheromone. Angelika therefore set up a highly ingenious scheme to identify temperature sensitive mutations that would permit a single cell cycle Start in the presence of pheromone at the restrictive temperature (as measured by activation of the HO gene) despite an intact signal transduction pathway as measured by induction of known pheromone inducible genes like FUS1. Angelika did indeed find such mutants and they fell into four Alpha factor Resistant Lethal complementation groups, which she called ARL1, 2, 3, and 4. She cloned ARL1 and found no homology to any other protein in the databases. Because the lethality of arl mutants did not arise from a defect in a specific phase of the cell cycle, she decided that they were not directly concerned with pheromone cell cycle arrest and studied the genes no further. At some later stage, Gustav Ammerer found that at least two of the genes encoded subunits of the RSC nucleosome remodelling complex. Indeed, a quick BLAST of the ARL1 sequence from Angelika’s Diplomarbeit reveals that it corresponds to RSC9. In hindsight, Angelika was correct not to pursue the mutants. RSC probably has no specific role in pheromone cell cycle arrest and has highly pleiotropic effects because it affected the transcription of numerous genes. Mutations affecting RSC presumably showed up in Angelika’s screen because they somehow affected transcription of her markers for cell cycle progression and pheromone signal transduction, namely simultaneous activation of the HO and FUS1 genes. As they say in genetics, “look and ye shall find”. We certainly got what we were looking for, but the problem was that we were looking in the wrong place. As it happens, Fred Chang working in Ira Herskowitz’s lab embarked on a similar screen with a key difference, namely they assumed that the sought after gene was non-essential and that it had eluded Hartwell merely because he had not identified sufficient numbers of mutants. By screening for cells capable of sustained proliferation to pheromone despite being capable of inducing FUS1, Fred identified a gene called FAR1 that had precisely the properties predicted by Hartwell. The moral of this story is that genetic screens are rarely saturated and that it always pays to revisit them with more powerful methods
Gallery: Angelika Amon and the IMP
It was clearly a disappointment for Angelika that Fred’s simpler approach had worked while hers had failed. Lee’s failure to identify FAR1 was not because it was an essential gene, which we had assumed, but merely that it was hidden among a plethora of genes concerned with signal transduction. In the long run, however, I think this failure, far from being detrimental, probably contributed in a positive way to Angelika’s development. Scientists operating on the frontiers of our knowledge must take risks that lead frequently to failure. Managing this failure is one of the keys to becoming a successful scientist. Learning how to deal with failure or discovering that one has the personality to do so is therefore a key skill or event in our education. This was brought home to Angelika at a very early stage in her career and I strongly suspect it served her well ever after.
Bouncing back from disappointment after "Annus Mirabilis" of cell cycle control
Resilience was another of Angelika’s talents and she soon bounced back from the disappointment of her Diplomarbeit. She stayed in my lab and embarked on an entirely new journey for her doctorate. 1988, the year Angelika arrived in my lab, had been an Annus Mirabilis for those interested in the control of cell division. It witnessed the discovery that the initiation of DNA replication and the onset of mitosis are regulated by a single highly conserved cyclin dependent protein kinase now known as Cdk1. In budding yeast, early and late cell cycle events were triggered by forms of Cdk1 that differed in their cyclin subunits. Cln cyclins triggered events leading to S phase while B-type cyclins known as Clbs triggered M phase. How cells produced successive waves of Cln/Cdk1 and Clb/Ckd1 kinase activity during each cell cycle and how the end of one cycle led inexorably to the beginning of another were considered questions of great importance. Indeed, they were questions central to understanding the feature of living organisms that distinguishes them from non-living systems, namely the ability to reproduce. It was to these questions that Angelika now turned her attention, initially in close collaboration with a postdoc Uttam Surana.

During the next three years, Angelika was the first author on four key papers that revealed ever greater insight into these questions. The four papers appeared one by one, with a relentless logic, like a slow burning fuse that gradually crept closer and closer to her final, most important, and indeed explosive discovery, that the onset of cyclin B proteolysis initiated during M phase remains active until activation of Cln/Cdk1 kinases in late G1 of the subsequent cycle. I rarely go back and read old papers from my own lab, either in the mistaken belief that I can remember most of the important details or because I do not wish to remind myself of the error of our ways or their insignificance when revealed by the passage of time. However, my recent re-reading of Angelika’s papers laying out the logic of Cln and Clb/Cdk1 waves has been a revelation. They reminded me of the clarity of her logic, the incisiveness of her questions, the virtuosity of her experiments, the clarity of her results (no ifs and maybes), and her relentless pursuit of the next obvious question. Her personality leaps off the page as one re-reads these papers. Even more important, one was reminded how central some of these papers were to the development of the whole field of chromosome biology. They were not dead ends!
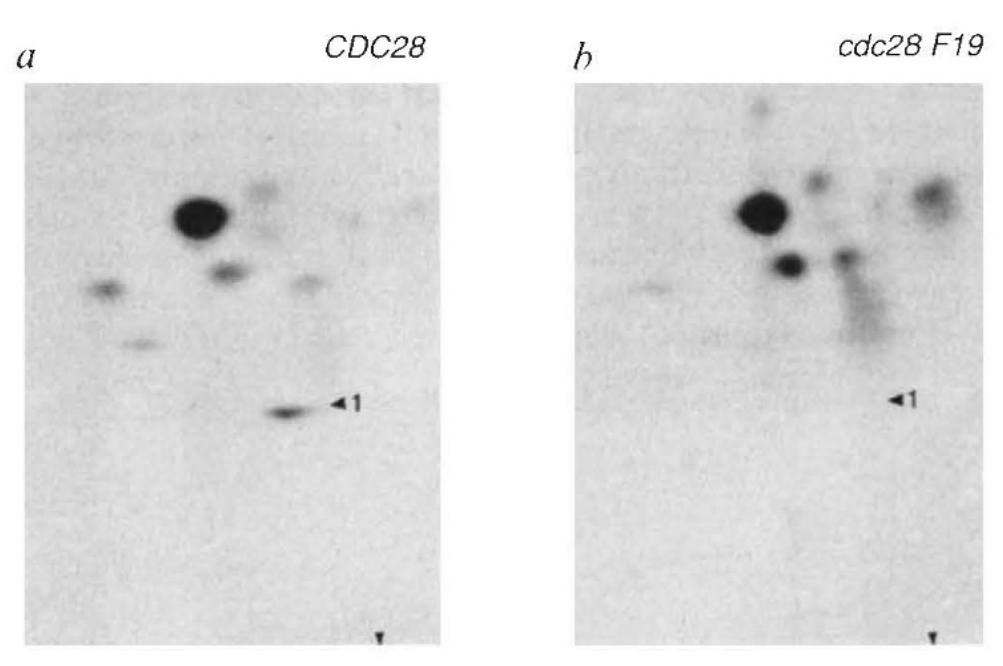
This journey started when Angelika turned from genetics to biochemistry. Beautiful work from Kathy Gould in Paul Nurse’s lab had shown that the transition from G2 to M phase in fission yeast (S. pombe) is accompanied by de-phosphorylation of a highly conserved tyrosine (Y15) in Ckd1 (Cdc2). Y15 is phosphorylated by Wee1 and de-phosphorylated by Cdc25 and replacement of the residue by phenylalanine causes cells to enter mitosis prematurely as occurs when Wee1 is inactivated. Angelika discovered that the equivalent residue in Cdc28, namely Y19, is also phosphorylated in a cell cycle dependent fashion, but unlike in fission yeast, its replacement by phenylalanine had little effect on cell cycle progression or in arresting the cell cycle in response to DNA damage. It turns out that while regulation of the G2 to M phase transition has a pivotal role controlling the onset of chromosome segregation in fission yeast and indeed in many other eukaryotes, regulation of the onset of anaphase is more important in budding yeast and this is the reason why CDC28Y19F has such a mild phenotype, at least in the laboratory. As it turns out, this fundamental difference was an important reason why Cdk1’s role in triggering mitosis was discovered in S. pombe while its nemesis, the Anaphase Promoting Complex, was discovered in S. cerevisiae. Publication of your first paper is an important “Rite de Passage”, whether or not it describes an important breakthrough. Angelika’s publication reporting the comparative unimportance of Y19 phosphorylation in blocking Cdc28 kinase activity signalled her coming of age as a scientist. She had tasted the apple, not of knowledge, but of discovery, and there was little stopping her thereafter.
Overturning a prevailing dogma
The onset of M phase regulated by Cdk1 phosphorylation is characterized by dissolution of the nuclear membrane, by chromosome condensation, and by the alignment of sister chromatids on mitotic spindles, all dramatic, almost wondrous, events in the life of a eukaryotic cell. However, even more extraordinary is the subsequent disjunction of sister chromatids at the metaphase to anaphase transition. It was the precision with which chromosomes are split and their two halves (chromatids) segregated to opposite poles of the cell during anaphase that led to Roux’s suggestion that the process might have something to do with segregating precise qualities to each daughter cell, a proposal that can be considered the birth of the chromosome theory of inheritance. Angelika’s second paper, another joint effort with Uttam that involved a key collaboration with Breck Byers, who was then spending a sabbatical at the IMP, addressed the part played by Cdk1 inactivation in this process. Their work overturned the prevailing dogma and pointed an accusing finger instead at the role of a protein that had been discovered by Breck’s lab, namely Esp1.
The story started with Tim Hunt’s discovery that B-type cyclins, whose activation of Cdk1 is essential for entry into mitosis, are very rapidly destroyed by proteolysis at the metaphase to anaphase transition. Tim had shown that B-type cyclins were rock stable during G2 and early M phases but became very unstable at the onset of anaphase. This was first observed during the cleavage divisions of sea urchins but was later confirmed in a wide variety of eukaryotes. Subsequent work by Andrew Murray and Michael Glotzer in Marc Kirschner’s lab demonstrated that proteolysis was accompanied by ubiquitinylation and depended on a small peptide motif called the destruction box within an unstructured N-terminal domain. Because expression of cyclins lacking their destruction motif blocked chromosome segregation and exit from mitosis, they had proposed that “the transition from metaphase to anaphase, which marks the end of mitosis, is induced by the degradation of cyclin”. The subsequent claim by Steve Reed’s lab that expression of non-degradable B-type cyclins also blocked anaphase in yeast appeared to confirm that cyclin destruction would prove to be a universal trigger for anaphase. Angelika and Uttam had meanwhile been carefully measuring Cdk1 activity associated with B-type cyclins during passage of wild type and mutant yeast cells through mitosis and had obtained results that did not fit this paradigm. As expected, they found that the drop in Cdk1 activity was indeed accompanied by cyclin degradation, but more surprisingly, both events lagged several minutes behind sister chromatid disjunction. Even more striking was their discovery of a cell cycle mutant, namely cdc15, in which sister chromatids disjoined fully to opposite poles of the cell without any apparent drop in Cdk1 activity.
Because this finding suggested that cyclin destruction was not necessary for anaphase in yeast, which was hard to reconcile with the work from Reed’s lab, Angelika decided to re-investigate what really happens when yeast cells express a non-degradable B-type cyclin. The results could not have been clearer. Sister chromatid segregation was unaffected but exit from mitosis was blocked and cells arrested with spindles resembling those in cdc15 mutants. In other words, cyclin destruction was clearly not necessary for the metaphase to anaphase transition, at least in budding yeast. Careful scrutiny of cell cytology revealed how others had come to the opposite conclusion. In addition to blocking exit from mitosis, the precocious expression of B-type cyclins also blocked budding and the extensive spindle extension inside a round unbudded cell resulted in the fully disjoined chromatid sets being pushed around the circumference of the cell until they bumped into each other. If one merely observed cells blocked for long periods, and failed to notice the circularity of their spindles, this gave the entirely incorrect impression that disjunction had never taken place.
Results in black and white, not in shades of grey
My experience as a scientist over the years has led me to be sceptical of any so called discoveries that report results predicted by theory. Many philosophers of science would regard this as heretical as it is widely accepted that science proceeds by formulating hypotheses and making predictions that turn out to survive future observations and experiments. Of course, theory is vital, as are predictions, but it is not widely appreciated that results that do not fulfil your predictions have far greater value than those that do. There are two very good reasons for this.

First and foremost, expectations inevitably bias our perception and unless one has conducted a supremely careful experiment and produced data of the utmost clarity there is always a danger that one prefers the expected conclusion from results that are in fact ambiguous or not “black or white”. The second reason is that scientific progress is in fact driven by the discovery that current explanations are insufficient, which forces one to postulate new theories, in other words, by realizing that the current facts or new results do not fit theory. A corollary is that if you are to discover the discrepancies vital for all true scientific progress, your theory must be so transparent and your experiments must yield results so clear that you are literally forced to accept that you were wrong. It must not be possible to fall into the temptation of believing the facts do not in fact refute your hypothesis or that your hypothesis can be rescued by some ad hoc re-adjustment. I would like to give Angelika considerable credit for this philosophical epiphany. Though it may seem like a small thing, her discovery that the consequences of non-degradable cyclin were not as predicted by theory was a good example of the principle I have outlined above. Moreover, the reason why she had drawn the correct (unexpected) conclusion while others had drawn the wrong (expected) one derived from an experimental virtuosity that produced results in black and white and not in shades of grey.
Angelika and Uttam’s next key finding was inspired by Breck, whose quiet, calm, and undemonstrative presence contrasted with that of Angelika and Uttam, who were frequently to be seen locked in heated discussions, not just in the IMP but also in Café Prückel to which they would often retire in the evenings. This charming café at Stubentor on the Ringstrasse, a bus ride down Landstrasse from the IMP, was also renowned as a pick up joint and I wonder what its clientele thought when they overheard Angelika and Uttam’s heated discussions about cell reproduction. Breck’s lab had shown that despite apparently failing to segregate chromatids, esp1 mutants nevertheless re-entered new cell cycles during which they re-duplicated their spindle pole bodies. Hence, their description as mutants with Extra Spindle Pole bodies (or ESP). His lab had in fact identified two such genes, ESP1 and ESP2. The reduplication of spindle bodies in esp mutants suggested that the mutant cells might exit from mitosis despite having failed to segregate chromosomes. Angelika and Uttam discovered that this was indeed the case. Cdk1 inactivation took place with kinetics similar to wild type and re-induction of G1 cyclins confirmed that esp1 mutant cells clearly re-entered the next cell cycle. Thus, not only was cyclin B destruction not required for anaphase but it was also insufficient. An important corollary was that ESP1 and not cyclin destruction might have a rather specific role in triggering anaphase. At the time, it was not known whether the lack of chromosome segregation in esp1 mutants was due to defective spindle forces or due to a lack of sister chromatid disjunction. Later work by Rafal Ciosk showed that it was the latter and revealed that Esp1 is essential for removing cohesin from chromosomes. Frank Uhlmann subsequently developed an assay for this process and thereby discovered that Esp1 encodes a protease that triggers anaphase by cleaving cohesin’s kleisin subunit.
The world cannot be explained by current knowledge
Angelika and Uttam were both dismayed that their paper was rejected by Cell and it appeared instead in the EMBO journal. I had a long correspondence about this with Marc Kirschner, who argued forcibly that showing others were wrong did not constitute an important discovery. Needless to say, we did not agree. As Francois Jacob so eloquently put it, the real driving force of science is the recognition that the world cannot be explained by current knowledge, which forces one to imagine new forces. By eliminating cyclin proteolysis as the anaphase trigger, Angelika and Uttam showed that there must be another force out there. Their work was an important step in the eventual identification of the real anaphase trigger, namely activation of Esp1 by the machinery responsible for cyclin destruction, the ubiquitin protein ligase known as the Anaphase Promoting Complex or Cyclosome (APC/C). It is a measure of Angelika’s humanity that she never resented Marc's criticism and she came to value him as a great scientist and colleague in Boston where she made her home. A fascinating footnote to this story emerged from subsequent work in Marc's lab by Olaf Stemmann who showed that in egg extracts phosphorylation of separase by Cdk1 contributes to separase inhibition prior to activation of the APC/C. So cyclin destruction does indeed contribute to anaphase onset, but this form of regulation is entirely lacking in yeast, explaining why cyclin destruction has no role in sister chromatid disjunction in this organism. I have dwelt on this phase of Angelika’s work not only because it had a huge impact on future studies in my laboratory but also because it provided a stepping stone to Angelika’s subsequent career. Her early work as a PI at MIT focused on the mechanism by which Cdk1 is eventually inactivated in budding yeast, elucidating the key part played by the Cdc14 phosphatase and its activation via two different pathways, the FEAR pathway involving separase (Esp1) and the mitotic exit pathway involving Lte1 and Cdc15.
By this stage, it had become clear that G1 cyclins such as Cln1 and Cln2 promoted Start and thereby at least indirectly S phase while B-type cyclins like Clb1 and Clb2 promoted entry into M phase. Angelika turned her attention to how yeast cells ensure that a wave of G1 cyclin/Cdk1 activity preceded that associated with B-type cyclins. In parallel with Fred Cross’s lab, we had shown that the late G1-specific burst of CLN1/2 transcription depends on Cdk1 activity, suggesting that the appearance of Cln1/2 in late G1 is generated by a positive feedback loop. What then causes these cyclins to disappear subsequently? By creating a conditional allele of CLB2, Angelika showed that the repression of CLN1/2 transcription during G2 was mainly due to Cdk1 activity associated with Clb1/2. Moreover, like Cln1/2 in G1, the rise in Clb1/2 during G2 depended on their own activity, revealing a second positive feedback loop. Though this work highlighted important aspects of the network regulating G1 and G2 cyclin transcription, it left unanswered how Cln/Cdk1 kinase activity sowed the seeds of their own destruction, by enabling the auto-activation of Clb/Cdk1 kinases, a feature vital for ensuring that Cln kinases must appear before Clb kinases. It also provided no clue as to how Clb/Cdk1 kinases led in turn to their own inactivation through destruction box-mediated Clb proteolysis, an event crucial for permitting the cycle to re-start.
Though this work was a collaboration with Mike Tyers, most of the experiments described in her cyclin transcription paper were conceived and performed by Angelika. One sees here for the first time features that would characterize her subsequent career. These include the clarity of her logic, the specificity of her questions, the mastery of the experimental technique necessary to answer them, the unambiguity of her answers, and the relentless progression by which answers to one question led in turn to new questions. In short, it revealed the exquisite virtuosity of her empirical approach. The paper was important because it was the first attempt to put together a serious narrative to explain alternate oscillations of G1 and G2 cyclins. Doing so revealed more clearly than hitherto the missing gaps in our understanding. It transpires that insight into how cyclin B/Cdk1 kinases promote their own destruction, as first observed in Xenopus extracts, has only emerged rather recently, from the identification of Clb/Cdk1 phosphorylation sites within the APC/C necessary for its ubiquitinylation of Clbs. Understanding what prevented Clbs from activating themselves during early G1 emerged more rapidly, partly from subsequent work by Angelika showing that the cyclin destruction machinery remains active during early G1 and is only turned off by Cln/Cdk1 kinases (see below) and partly from work by Etienne Schwob and Mike Mendenhall showing that Clb/Cdk1 inactivation at the end of mitosis triggers the accumulation of a specific Clb/Cdk1 kinase inhibitor called Sic1 whose later destruction (at the hands of the SCF ubiquitin protein ligase) necessary for Clb/Cdk1 activity, is triggered by Cln/Cdk1 kinases.
Wrapping up in Vienna...
Angelika’s last and most important piece of work in my lab followed directly from her studies on Cln/Clb oscillations. It started by asking how cells ensure that the Clb/Ckd1 auto-activation loop is not triggered in G1 cells. To address this, she analysed the consequences of transcribing CLB2 in cells arrested in G1 due to depletion of Cln cyclins. She showed that little or no Clb2 protein appeared despite efficient accumulation of CLB2 mRNAs, that destruction box-mediated proteolysis was responsible, and finally that Cln/Cdk1 kinase activity was required to shut off this proteolytic pathway. This revealed that proteolysis of Clb cyclins initiated during mitosis remains active during the subsequent G1, thereby preventing the premature accumulation of G2 cyclins, and that it is only shut off upon the activation of Cln/Cdk1 kinases. Thus, one of the reasons why Clns appear before Clbs is that they are not degraded by the destruction box pathway and can therefore accumulate even when this pathway is fully active. It is remarkable that most of the experiments described in her proteolysis paper were conducted after Angelika had formally left my lab and had started working as a postdoc in the botany department with Dieter Schweizer, prior to her move to Boston. She would put in a full day in Dieter’s lab and then hop back on the 71 tram for a spot of moonlighting at the IMP.
Angelika’s paper describing this phenomenon was of immense importance as it opened the door to using genetics to identify genes involved in B-type cyclin proteolysis. Angelika had shown that destruction box-mediated B-type cyclin proteolysis is shut off by Clns in late G1 and remains inactive until the onset of anaphase. Thus, any screen that searched for temperature sensitive mutants defective in destruction box-mediated proteolysis would be confounded by a plethora of mutants that appeared defective in proteolysis merely because the cells arrested in G2 or M phase, when the destruction box pathway is naturally inactive. Angelika’s work provided a solution to this problem, namely to conduct the screen in cells that could be uniformly arrested in early G1 and pick mutants that could accumulate a Clb2-LacZ fusion protein after cells had been arrested in G1 due to Cln depletion. Stefan Irniger subsequently conducted this screen and his work led to the identification of Cdc16 and Cdc23 as key proteins, a discovery that led directly to their identification as subunits of the APC/C. Indeed, it was Stefan’s discovery that the cyclin destruction machinery was essential for the metaphase to anaphase transition that led to the ubiquitin protein ligase being named the Anaphase Promoting Complex as well as the Cyclosome. Because CDC16 and CDC23 were necessary for anaphase while cyclin B proteolysis was not, Stefan predicted the existence of an anaphase inhibitor that was degraded by the APC/C at the same time as cyclins, an idea that had also been postulated by Sandra Holloway in Andrew Murray’s lab. The identity of this inhibitor, now known as securin, emerged soon thereafter from work in Doug Koshland's and Mitsohiro Yanagida’s labs.
...before departing for Boston
Angelika did not participate directly in the screen conducted by Stefan as she had by this stage departed for Boston and her moonlighting operation was no longer feasible. However, soon after setting up her own lab at the Whitehead, she made the very important finding that the proteolysis of securin at the metaphase to anaphase transition and the continued proteolysis of B-type cyclins during the subsequent G1 were mediated by different forms of the APC/C, the former by APC/C associated with Cdc20 while the latter by APC/C associated with its homologue Cdh1. Subsequent work by Wolfgang Zachariae in my lab in collaboration with Wolfgang Seufert showed that Cdh1 is inhibited by phosphorylation by Cdk1, ensuring that it is only active during early G1. It is ironic that Stefan’s genetic screen had not pinpointed Cdh1’s role as he had focused only on mutations that caused lethality, an error that I suspect Angelika would not have made given her arl debacle.
I have dwelled on this aspect of Angelika’s work for two reasons. By facilitating the identification of the APC/C, her work not only had a huge impact on the entire cell cycle field but also altered the entire course of my career. If we had not discovered that the cyclin proteolysis machinery, albeit not the destruction of cyclins per se, was required for the metaphase to anaphase transition, then we would never have embarked on our search for mutants defective in sister chromatid cohesion, on the mistaken assumption that cohesion proteins might be targets of the APC/C. In other words, we would never have participated in the discovery of cohesin, on which my lab has focused ever since and which has been a magical odyssey, if not obsession. Angelika says that she was inspired to study cell biology by watching old movies of sister chromatid disjunction at the metaphase to anaphase transition. Was it merely a coincidence or was it fate that led her to make such a remarkable contribution to understanding this extra-ordinary process?

It is a tragedy that Angelika has departed, not only because we will greatly miss her but also because she was at the very peak of her scientific career and had so many more key discoveries to make. Others may pick up the reins where she has let go, but progress will be slower without her indomitable and relentless truth seeking. She was a rare scientist who fully satisfies the subtraction test, namely would her absence have made a difference. It sure would have and still will. Despite this tragedy, I feel a small measure of comfort that even during her all too short presence she created intellectual waves that will continue to oscillate in her absence. She created as it were many ripples of knowledge within a sea of ignorance and it will be some time before these reach our shores. The consequences of her virtuoso experimentation, her inquisitive imagination, and her unique personality will continue with us for some time and in this sense her spirit lives on. I was extra-ordinarily lucky that she got on the 71 tram and sought me out as she changed and enriched my life forever.
Published in November 2020
Further reading
Obituary: Angelika Amon (1967 to 2020)









