How cohesin regulates the genome by loop extrusion?
Cohesin was discovered for its ability to connect replicated DNA molecules, an activity that is essential for chromosome segregation in proliferating eukaryotic cells. Through our discovery of two cohesin regulators, called WAPL and CTCF (Kueng et al., Cell 2006; Wendt et al., Nature 2008), we realized that cohesin has an even more universal role, namely in folding genomic DNA in both proliferating and non-proliferating cells. We showed that cohesin accumulates at the same genomic positions as CTCF and is required for its transcriptional insulator function (Wendt et al., Nature 2008). Because this function was thought to depend on chromatin looping, we reasoned that cohesin might not only connect DNA molecules in trans to mediate cohesion but also in cis to form loops (Wendt et al., Nature 2008; Wendt & Peters, Chrom. Res. 2009). We and others showed that this is indeed the case (Hadjur et al., Nature 2009; Nativio et al., PLoS Gen. 2009; Seitan et al., Nature 2011; Gassler et al., EMBO J. 2017; Rao et al., Cell 2017; Schwarzer et al., Nature 2017; Wutz et al., EMBO J. 2017).
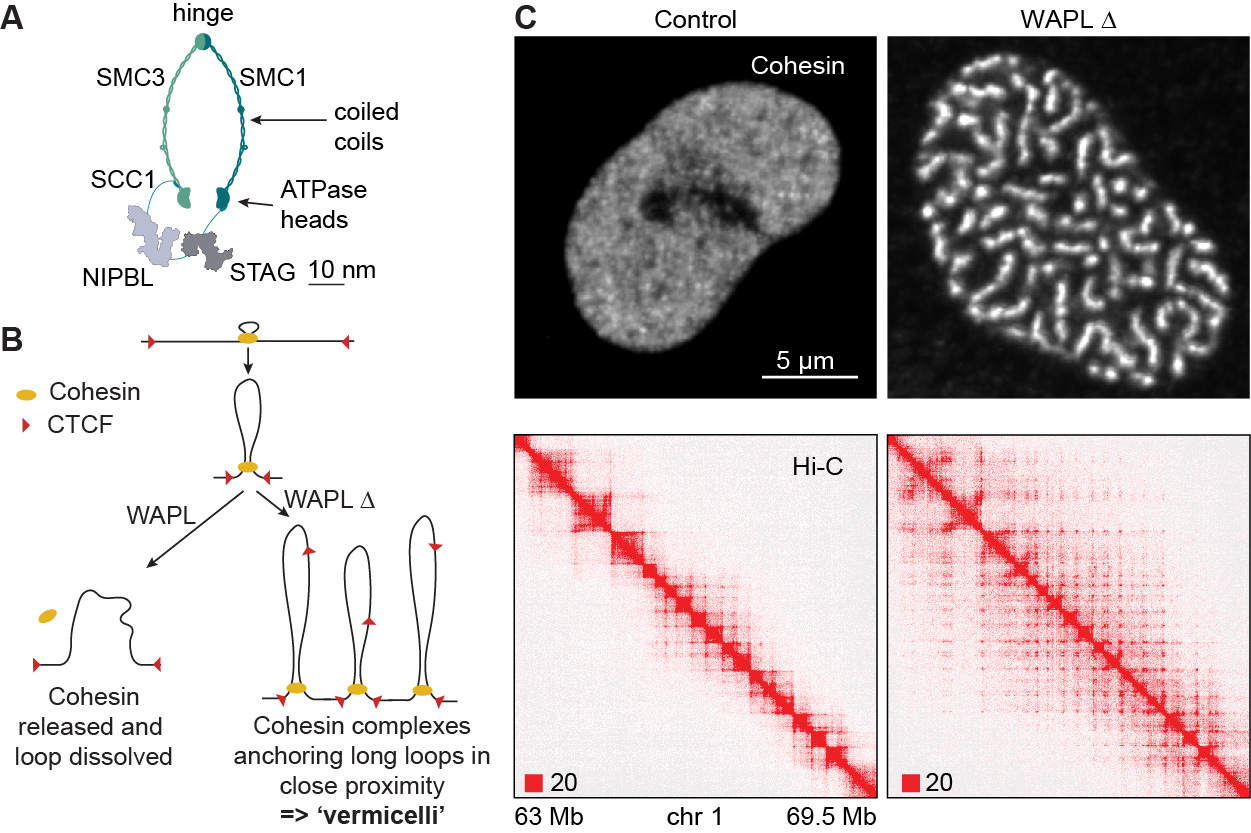
We found that WAPL releases cohesin from interphase chromatin (Kueng et al., Cell 2006), and thereby limits the length and lifetime of chromatin loops (Wutz et al., EMBO J. 2017; see also Haarhuis et al., Cell 2017). We also made the unexpected observation that WAPL depletion relocates cohesin from most chromatin regions into axial chromosomal domains and proposed that these “vermicelli” represent regions in which cohesin anchors chromatin loops (Tedeschi et al., Nature 2013).
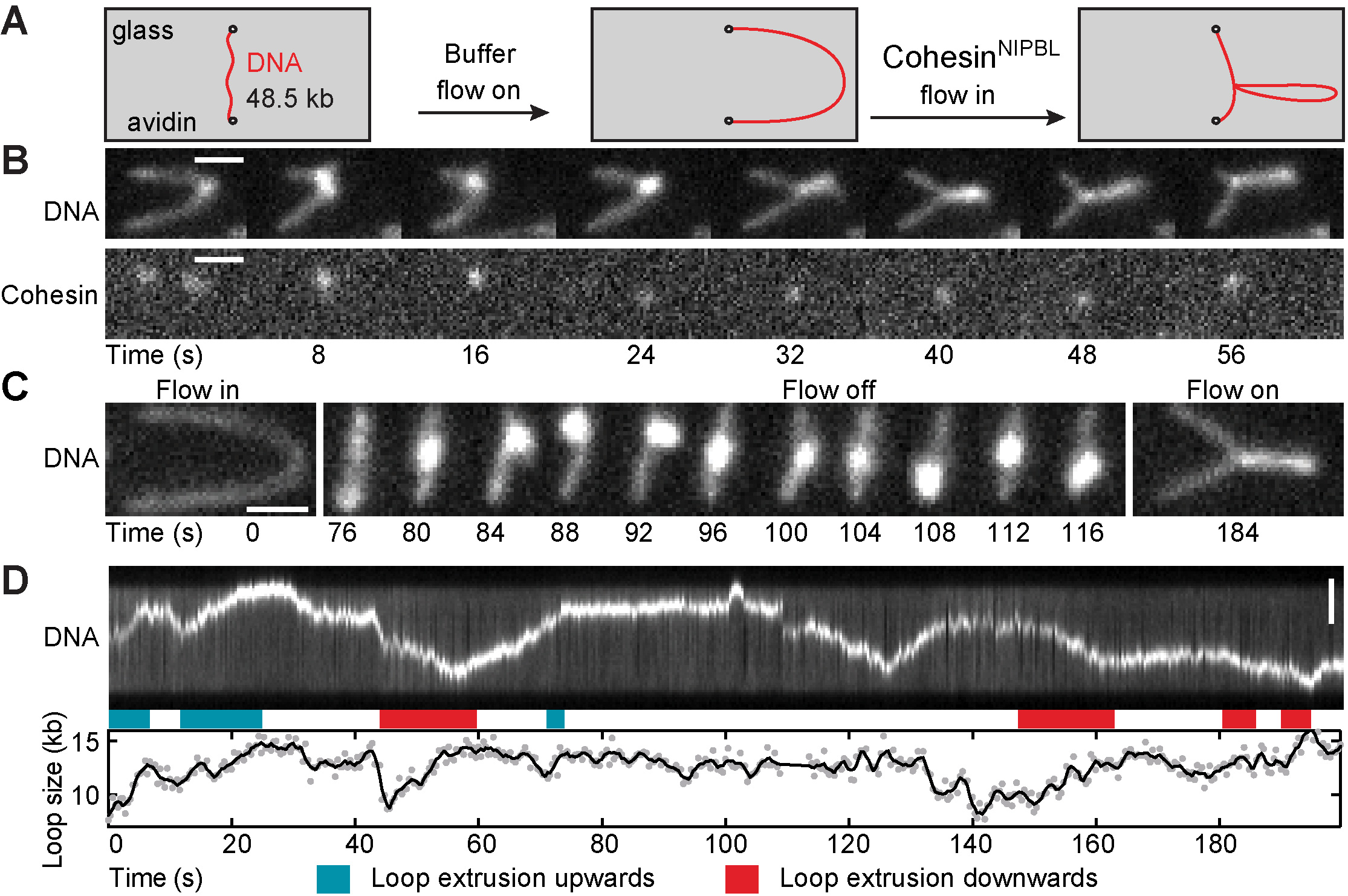
Since this phenomenon could only be explained by the then controversial and largely untested loop extrusion hypothesis (Nasmyth, Ann. Rev. Gen. 2001; Nichols and Corces, Cell 2015; Sanborn et al., PNAS 2015; Fudenberg et al., Cell Rep. 2016), we directly tested this hypothesis through biochemical reconstitution and single-molecule imaging. These experiments showed that cohesin rapidly extrudes DNA into loops in the presence of a protein called NIPBL (Davidson et al., Science 2019; for similar findings, see Kim et al., Science 2019; Brugues et al., eLife 2020). Until then, cohesin had for almost two decades been thought to be a passive DNA linker and NIPBL a cohesin loader that is no longer needed once cohesin has bound to DNA. Instead, our results indicate that cohesin is a motor protein and that NIPBL is a processivity factor for loop extruding cohesin.
Video 1. TIRF microscopy video visualizing cohesin-mediated loop extrusion of fluorescently labeled DNA under buffer side-flow conditions (Davidson et al., Science 2019).
What are the functions of loop extrusion?
The finding that genome folding is an active and tightly controlled process implies that cohesin-mediated loop extrusion serves important functions. We suspect that some of these functions are as ancient as DNA genomes themselves, since cohesin is a member of the structural-maintenance-of-chromosomes (SMC) family of ATPases, which fold DNA in all kingdoms of life. SMC complexes might have evolved early in bacteria to facilitate segregation of their circular genomes (reviewed in Davidson & Peters, NRMCB 2021). But eukaryotic SMC complexes have adopted important additional functions. For example, cohesin-mediated loop extrusion is required for V(D)J recombination in developing B cells to generate the antibody repertoire that is needed for protection from pathogens (Zhang et al., Nature 2019; Hill et al., Nature 2020; see website of Meinrad Busslinger’s group at the IMP for more information). Similarly, we found that cohesin complexes are required for the formation of long enhancer- promoter loops, whereas short enhancer-promoter interactions can be generated without cohesin (Thieke et al., Cell Rep. 2020). Recently, we discovered that cohesin contributes to the genomic positioning of the epigenetic reader protein Phf2, suggesting that cohesin-mediated loop extrusion also contributes to epigenetic regulation of chromatin (Tang et al., EMBO J. 2025). We are analyzing these and additional functions of cohesin-mediated loop extrusion through genetic, cellular and genomic approaches.
How is loop extrusion regulated?
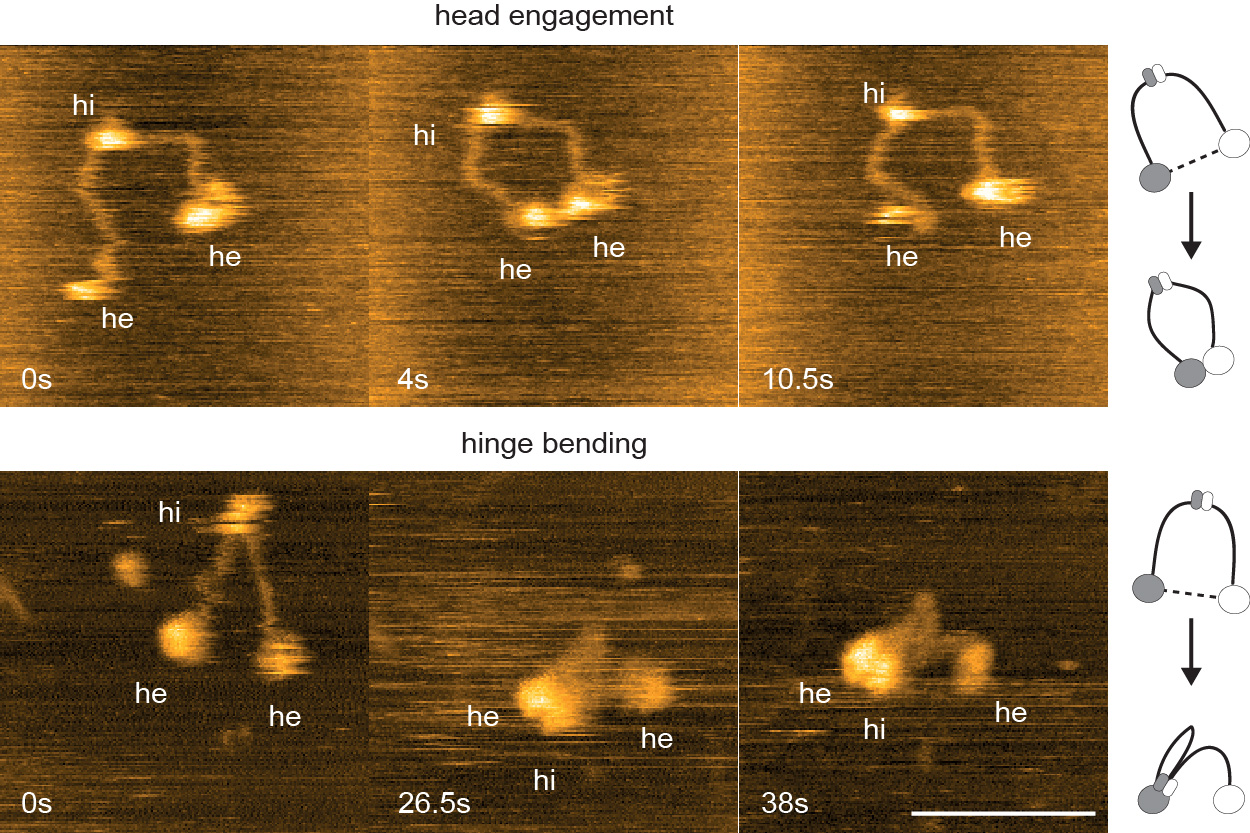
Cohesin-mediated loop extrusion must be tightly controlled to allow gene regulation and recombination. Our work has shown that CTCF and WAPL are key for this regulation. CTCF functions as the main genomic boundary for cohesin and thus positions loops on DNA (Wendt et al., Nature 2008; Busslinger et al., Nature 2017; Wutz et al., EMBO J. 2017; Davidson et al., Nature 2023; see also Nora et al., Cell 2017; Zhang et al., Mol. Cell 2023). But active genes (Banigan et al., PNAS 2023) and the replicative MCM helicase (Dequeker et al., Nature 2022) also contribute to this spatial regulation. WAPL limits the residence time of cohesin and thus the length and lifetime of loops (Kueng et al., Cell 2006; Tedeschi et al., Nature 2013; Wutz et al., EMBO J. 2017 see also Haarhuis et al., Cell 2017) but is transcriptionally repressed in developing B cells and neurons to mediate gene recombination and gene regulation through the formation of exceptionally long cohesin loops (Hill et al., Nature 2020; Dai et al., Nature 2021; Kiefer et al., Science 2023). Cohesin is also regulated by PDS5 proteins (Wutz et al., EMBO J. 2017; Wutz et al., bioRxiv 2025), which can replace NIPBL on cohesin but cannot support loop extrusion (Davidson et al., Science 2019). We are currently analyzing how WAPL, PDS5 and other proteins control cohesin in different cell types, including developing B cells.
What is the mechanism of loop extrusion?
How cohesin and other SMC complexes translocate DNA into loops is poorly understood (Dekker et al., Science 2023). Our work indicates that this process depends on large-scale conformational changes in cohesin that are controlled by cohesin’s ATP binding-hydrolysis cycle (Bauer et al., Cell 2021). We are using biochemical, structural and single-molecule biophysics techniques to understand how cohesin’s conformational changes reel DNA into loops. We have recently found that cohesin supercoils DNA during loop extrusion (Janissen et al., Sci. Adv. 2024; Davidson et al., Cell Rep. 2025). We are therefore exploring whether resolution of supercoils by topoisomerases is required for the formation of cohesin loops. Since our previous in vitro reconstitutions have analyzed how cohesin extrudes “naked” DNA (Davidson et al., Science 2019; Davidson et al., Nature 2023; Barth et al., Cell 2025) we are now addressing how cohesin extrudes DNA that has been assembled into chromatin.
Video 2: Hypothetical loop extrusion mechanism. (for more details, see Dekker et al., Science 2023)
How do cohesin mutations contribute to congenital diseases and cancer?
Our findings have potentially important implications for understanding diseases that are associated with cohesin mutations, such as “cohesinopathies” and cancers. We found that inactivation of STAG2, a cohesin subunit frequently mutated in several human cancers, changes genome architecture (Wutz et al., eLife, 2020; see also Kojic et al., NSMB 2018; Viny et al., Cell Stem Cell 2019; Casa et al., Genome Res. 2020). We also showed that some of the NIPBL mutations associated with the congenital disease Cornelia de Lange Syndrome cause defects in loop extrusion (Panarotto et al., PNAS 2022). These observations suggest that defects in cohesin-mediated loop extrusion contribute to the etiology of these diseases. We have also discovered a strong synthetic lethality between mutation of STAG2 and loss of its paralog STAG1, suggesting that STAG1 inactivation will kill STAG2 mutant tumor cells with high efficacy and specificity (van der Lelij et al., eLife 2017; van der Lelij et al. Life Sci. All. 2020). We are therefore exploring the effects of cohesin mutations and the potential suitability of STAG1 as a cancer drug-target through genetic, cellular and chemical approaches.
Selected Publications
- Wutz, G., Davidson, I.F., Banigan, E.J., Kawasumi, R., Stocsits, R.R., Tang, W., Nagasaka, K., Costantino, L., Jansen, R., Hirota, K., Branzei, D., Mirny, D. and Peters, J.-M. (2025). PDS5 proteins control genome architecture by limiting the lifetime of cohesin-NIPBL complexes. bioRxiv, doi.org/10.1101/2025.08.30.673243
- Tang, W., Costantino, L., Stocsits, R., Wutz, G., Ladurner, R., Hudecz, O., Mechtler, K. and Peters, J.-M. Cohesin positions the epigenetic reader Phf2 within the genome (2025). EMBO J., 2025 Jan 2.doi: 10.1038/s44318-024-00348-2, online ahead of print.
- Davidson, I.F., Barth, R., Zaczek, M., van der Torre, J., Tang, W., Nagasaka, K., Janissen, R., Kerssemakers, J., Wutz, G., Dekker#, C. and Peters#, J.-M (2023). CTCF is a DNA-tension-dependent barrier to cohesin-mediated DNA loop extrusion. Nature 616, 822-827. doi: 10.1038/s41586-023-05961-5. Epub 2023 Apr 19. PMID: 37076620
- Bauer, B., Davidson, I.F., Canena, D., Wutz, G., Tang, W., Litos, G., Horn, S., Hinterdorfer, P. and Peters, J.-M. (2021). Cohesin mediates DNA loop extrusion by a “swing and clamp” mechanism. Cell 184, 5448-5464. doi: 10.1016/j.cell.2021.09.016. Epub 2021 Oct 7.
- Davidson, I.F., Bauer, B., Goetz, D., Tang, W., Wutz, G. and Peters, J.-M. (2019). DNA loop extrusion by human cohesin. Science 366, 1388-1345.
Join us
- Master students and Post-docs: Contact Jan-Michael Peters with a letter of intent detailing why you want to join the lab.
- PhD students: Calls open 1 March and 1 September, apply here:
Vienna BioCenter PhD Program

