The dormant egg: scientists bring the ‘sleep mode’ of egg ribosomes to light
In animals, eggs serve as a stockpile that contains all the necessary components for the early stages of embryonic development. Ribosomes, the molecular machines that produce proteins, are deposited in large amounts in the egg, yet most of them remain inactive to preserve energy until fertilisation and first cell divisions. In a cross-disciplinary effort, scientists at the IMP have discovered the molecular mechanism that keeps ribosomes dormant in fish and frog eggs and report their findings in the journal Nature.
Most cells in the adult body perform their functions with the help of a vast number of proteins that they produce from mRNA templates using large molecular ‘machines’, called ribosomes. The mature egg is a special case: while it is filled with many mRNAs and ribosomes, its protein production is largely repressed. mRNAs and ribosomes are stored in the egg by the mother for later use in the embryo where they sustain its first cell divisions.
Before fertilisation occurs, the egg is in a dormant state: it maintains a low metabolism to save on energy and to protect important molecules from damage. One of these important molecules is the ribosome itself: Making these miniature machines requires a lot of energy, and they are one of the cell’s biggest energy consumers when they are active. Over the past decades, mechanisms controlling translation in the egg have been described for almost all components except for the core machinery, the ribosome.
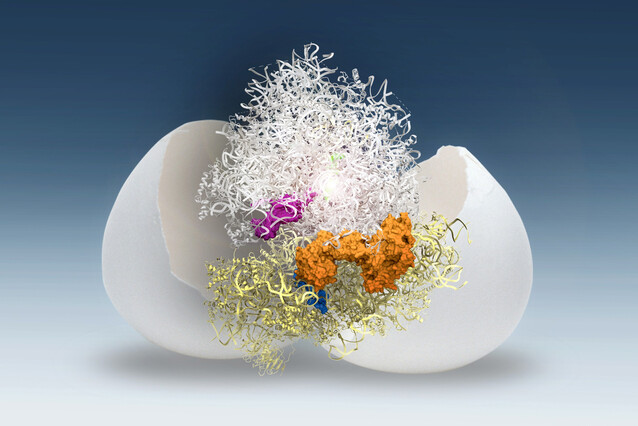
Researchers at the IMP, led by the labs of Andrea Pauli and David Haselbach, have investigated the molecular mechanism that keeps the ribosome in ‘sleep mode’ in zebrafish and frog eggs. Because ribosomes have a limited lifespan, preserving their functionality by keeping them in a dormant state in the egg is advantageous for the embryo. The team identified two modules of two molecules each that bind ribosomes and keep them stable and inactive in the egg. Their findings are now published in the journal Nature.
Four dormancy molecules to keep ribosomes safe and silent
“In the early days of my doctoral studies, I used cryo-electron microscopy on zebrafish egg ribosomes and looked closely at 3-D reconstructions. I noticed that something was stuck in the ribosome’s exit tunnel. That’s where newly formed proteins normally emerge. At first, I thought a protein was being synthesised and I couldn’t believe it – translation isn’t supposed to be very active in eggs,” says Frieda Leesch, PhD student and co-first author. “We discovered that this mysterious structure was in fact a previously unknown molecular ‘plug’ that stalls the ribosome.”
After further investigation, the scientists determined that this ‘plug’ was the small protein Dap1b, which had never been associated with the ribosome before. Working together with three other proteins, Dap1b prevents ribosomes from kicking off the process of translation.
Using genetic methods, the scientists determined that one module of two proteins stabilises ribosomes, while the second module, which includes Dap1b, inhibits ribosomal activity (see figure below). Experimentally removing these factors in fish eggs destabilises ribosomes, pinpointing them as crucial for ribosome maintenance.
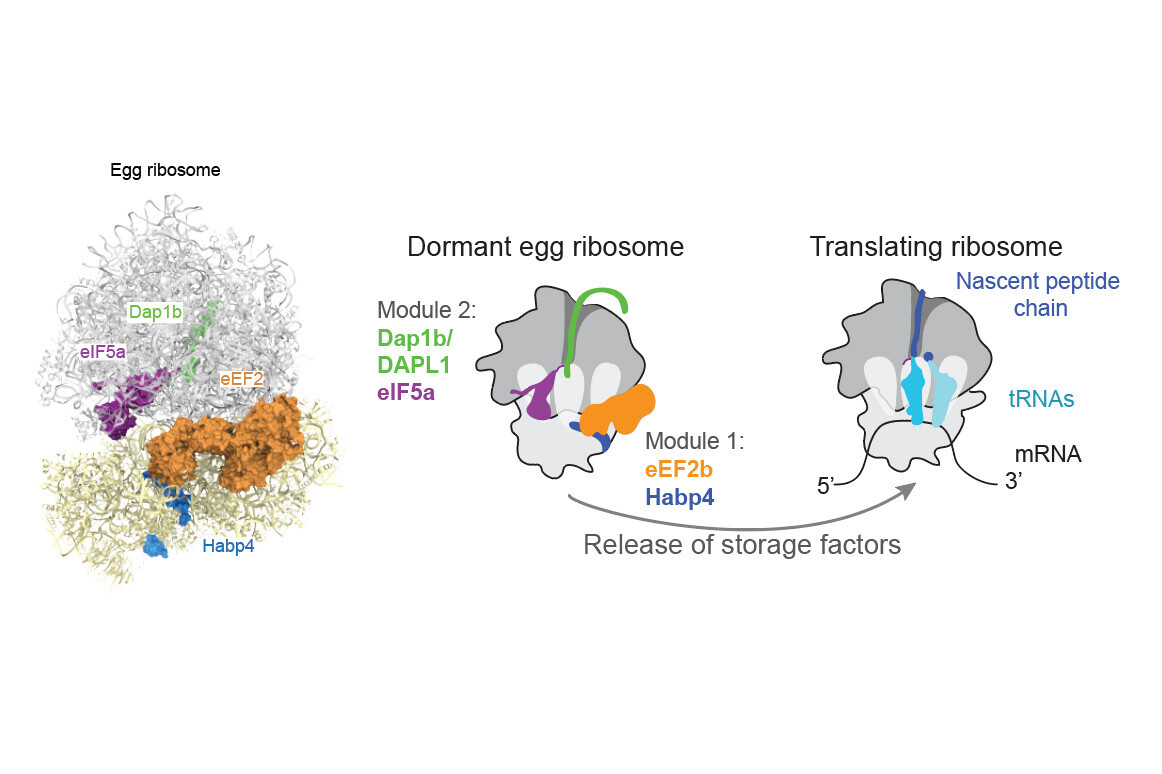
“We ran experiments in zebrafish and in frogs and identified the same set of molecules in dormant ribosomes. Using in vitro experiments, we even showed that adding Dap1b to mammalian ribosomes is enough to repress translation,” says Laura Lorenzo-Orts, postdoc and co-first author. “This basic molecular principle of ribosome dormancy could be the same in many other cell types and other vertebrates, including humans.”
Cross-disciplinary approach solves long-standing mystery
Several labs and facilities contributed to making this all-IMP study a success: in addition to Pauli’s expertise in developmental biology, David Haselbach’s lab and Anton Meinhart from the lab of Tim Clausen helped visualise egg ribosomes with cryo-electron microscopy. They also contributed to building 3D models of the dormancy factors to pin down their location on the ribosome. Moreover, the IMP’s protein chemistry facility helped identify all the proteins that are bound to egg ribosomes. Finally, Tzi-Yang Lin from the lab of Elly Tanaka brought in his knowledge of frog cell biology to expand the findings to a different vertebrate.
“This fundamental discovery results from a spur-of-the-moment discussion that I had with David Haselbach five years ago. Our findings required an interdisciplinary effort combining developmental biology, genetics, structural biology, and biochemistry. I am very proud of my group and our collaborators for bringing our ideas to fruition,” explains Andrea Pauli. “The next steps in this research will be to find out how the factors are released from the ribosome to activate translation in the early embryo, and whether the mechanism that we discovered in eggs acts in other cells of the body.”
“Biologists have researched egg dormancy for decades but had not figured out how their ribosomes are kept silent. One of the strengths of the IMP is its culture of collaboration – and it paid off for us,” says David Haselbach. “Bridging the world of individual molecules with the macroscopic world of phenotypes is the task of molecular biology. However, structural and in vivo phenotypic experiments in vertebrates are rarely brought together in a single study. The modern structural biology set-up at the IMP allows to bring these separate worlds closer together and gives researchers of all fields easier access to it.”
Scientists are just starting to understand the unique cellular environment of the egg. The dormant egg challenges textbook knowledge: characterising cellular and molecular conditions at the beginning of life will help gain a new perspective on fundamental biological processes, such as translation.
Original publication
Friederike Leesch*, Laura Lorenzo-Orts*#, Carina Pribitzer, Irina Grishkovskaya, Josef Roehsner, Anastasia Chugunova, Manuel Matzinger, Elisabeth Roitinger, Katarina Belačić, Susanne Kandolf, Tzi-Yang Lin, Karl Mechtler, Anton Meinhart, David Haselbach#, Andrea Pauli#: “A molecular network of conserved factors keeps ribosomes dormant in the egg”. Nature (2023), DOI: 10.1038/s41586-022-05623-y.
*These authors contributed equally to this study.
# co-corresponding authors
Further reading