How do human antibodies get better at fighting infections?
A team of researchers from the Pavri lab at the IMP have discovered how B cells organize the genes and regulatory regions involved in somatic hypermutation, a key process for producing protective antibodies after infection or vaccination. Their findings challenge previous models of how chromatin structure regulates antibody maturation and may have important implications in understanding the origin of certain immune disorders associated with poor antibody responses.
In this interview, co-first authors Ursula Schöberl, Johanna Fitz and Maximilian von der Linde discuss their findings, as well as how the IMP’s scientific culture and support enabled their research.
Can you summarize your main finding?
Ursula Schöberl: Our research focuses on how B cells fold their DNA in three dimensions to enable long-range interactions between genes and regulatory regions during antibody maturation. For the first time, we studied how somatic hypermutation and class switch recombination – two essential processes for antibody maturation – happen concomitantly in human B cells. Using a specialized method called Tri-C, we mapped the main DNA interactions occurring during somatic hypermutation and class switch recombination and found that the DNA can form a flexible structure to accommodate both processes without them competing.
What is antibody maturation and why is it essential to our immune system?
Ursula Schöberl: When our immune system recognizes a foreign, potentially dangerous particle, an antigen, a coordinated immune response is launched. Essential to this response are B cells, which produce antibodies that bind to the antigen, helping to neutralize and eliminate the potential threat. After the initial response, B cells try to improve and diversify their antibodies to ensure that the antigen is eliminated efficiently—especially if it reappears in the future.
This is a two-pronged process: on one hand, B cells use somatic hypermutation to deliberately mutate the DNA sequences encoding the antibody’s variable region, generating new antibody variants and selecting those that best recognize the antigen. This evolution-like process is essential to help our immune system defend against infections and develop protective immunity after infection and vaccination.
At the same time, B cells employ class switch recombination to replace the DNA segment encoding the antibody’s constant region. By switching to different constant regions, B cells can produce distinct antibody classes with specialized functions – such as acting as surface receptors helping attract other specialized immune cells to eliminate the pathogen.
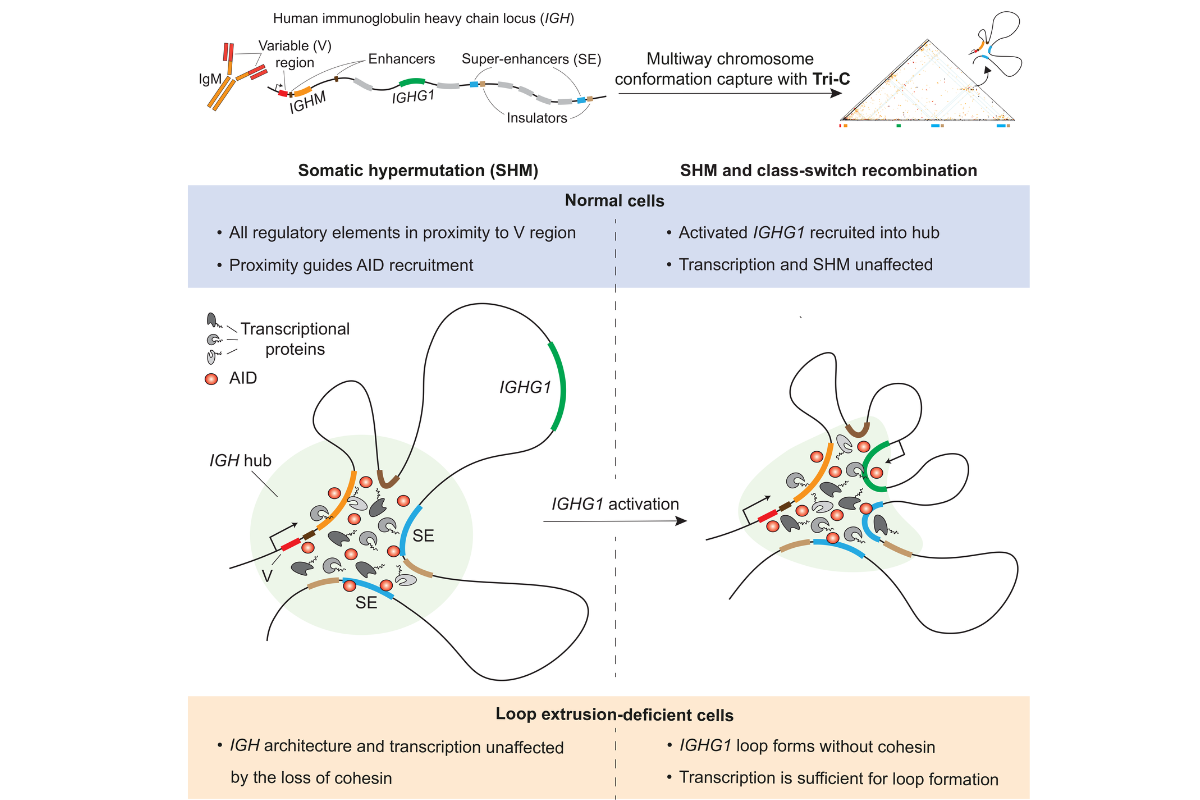
Can you tell us more about Tri-C, the technology that enabled these findings?
Maximilian von der Linde: Common methods can only detect interactions between two DNA regions, which limits our understanding of the complex three-dimensional interactions between multiple genes and enhancers that occur during antibody maturation. To overcome this issue, we used Tri-C, a method that allows us to study three-way interactions and infer how up to four different DNA regions come together.
Ursula Schöberl: In 2023, we used Tri-C in mouse B cells to study three-way interactions between DNA regions during class switch recombination, and we revealed how the DNA forms loops that bring distant DNA segments together to produce different classes of antibodies with unique functions. However, two major questions were unanswered: since mouse B cells do not undergo efficient somatic hypermutation in vitro, we could not study the role of chromatin structure in regulating this critical process. Consequently, we could not address whether somatic hypermutation and class switch recombination were competitive or compatible.
In our new article, we address these questions by using Tri-C in human B cells undergoing somatic hypermutation and combining this with a variety of genetic and molecular tools. This has been a longstanding goal for us and for the field and it’s very rewarding to have accomplished this.
How do your findings change our understanding of antibody maturation?
Johanna Fitz: Until now, we thought this was a rigid process driven by the molecular motor cohesin constantly pulling on the DNA to maintain a chromatin structure that traps genes and enhancers together. Our data shows that this structure can be maintained even without cohesin, challenging the previous models of antibody maturation. It was remarkable to uncover that losing cohesin didn’t affect this basic structure, and that transcriptional events didn't interfere in the interactions between DNA regions. This shows that the 3D organization of the antibody’s competent locus is very robust, opening exciting possibilities to therapeutically alter antibody maturation.
Ursula Schöberl: Another key takeaway is that proximity between genes and enhancers is vital to facilitate somatic hypermutation and class switch recombination. Our findings show that even though these processes are regulated at various levels, with many different proteins and DNA sequences involved, somatic hypermutation and class switch recombination can still happen together in the same nuclear space or hub without interfering with each other. This really changes the way we understand the role of DNA topology in antibody maturation and led us to propose a new model for the field.
Why is understanding antibody maturation important?
Ursula Schöberl: Many people suffer from immunodeficiencies caused by defects in antibody maturation, especially class switch recombination and antibody production. These individuals can’t produce certain types of antibodies or produce too few, compromising their immune system. By studying the mechanisms underlying antibody maturation, we can understand why these defects occur and work towards treatments to restore normal immune function in these patients.
Maximilian von der Linde: Our findings suggest that DNA conformation really matters for biologically relevant functions – and could inform research in other fields. We believe that mechanisms where changes in DNA topology are an essential layer of gene regulation worth studying not only in the genes involved in antibody maturation, but also in other loci across the genome.
How did your interdisciplinary expertise contribute to this project?
Johanna Fitz: This was a very rewarding collaboration between people with different backgrounds. For us wet lab scientists if was fantastic to have Max, our bioinformatician, literally sitting right next to us, and being able to constantly bounce ideas off each other and develop hypotheses, which greatly benefited the project.
Ursula Schöberl: A key factor was having worked together for many years. Even after the Pavri lab at the IMP closed and the three of us ended up at different institutions, we kept working closely together—including fun virtual meetings—and following our curiosity. And it paid off!
How did the IMP help you complete this project in these unusual circumstances?
Maximilian von der Linde: The IMP’s support was absolutely essential. It granted us continued access to its state-of-the-art resources, like the Vienna BioCenter’s computing cluster, even two years after officially leaving the institute. This, combined with the support of our new institutions and our own tenacity, allowed us to seamlessly complete our work.
Ursula Schöberl: The IMP extended my contract even after the Pavri lab was closed, allowing me to finish my experiments in-house and focus on what matters most—science. This support was instrumental in finishing the project.
Johanna Fitz: The infrastructure at our campus enabled us to perform the best science we could dream of. The Vienna Biocenter Core Facilities and the core IMBA/IMP facilities supported us with flow cytometry analysis, intense computing, and a considerable amount of next generation sequencing and molecular biology reagents, which were indispensable to this project.
What was the most rewarding aspect of working in this project?
Johanna Fitz: This is a prime example of curiosity-driven research. We spent years gathering data and collaborating across different institutes and even time zones with the shared interest of understanding how antibody maturation works. It was a fun ride and really fulfilling to see what we have accomplished.
Ursula Schöberl: Our project is a testament to the power of collaboration. Within our team, we supported each other every step of the way. Our collaborators Anton Goloborodko, Israel Tojal da Silva and Marta Rizzi and their students were also essential to this project, as were colleagues within the Vienna BioCenter and beyond, who provided invaluable insights and advice. We’re very grateful to them all and excited to keep collaborating in the future!
Original Publication
Ursula Schöberl*, Johanna Fitz*, Maximilian Christian von der Linde*, Renan Valieris, Bernd Bauer, Daniel Malz, Kimon Froussios, Julia Costea, Franziska Schmidt, Jeremy Pflaum, Bianca Bartl, Johanna Albrecht, Eva-Maria Wiedemann, Adriana Cantoran Garcia, Mihaela Peycheva, Marta Rizzi, Anton Goloborodko, Israel Tojal da Silva & Rushan Pavri#. Regulation of somatic hypermutation by higher-order chromatin structure. Molecular Cell, DOI: 10.1016&j.molcel.2025.06.003
*Co-first authors.
#Corresponding author.
Further reading
About the IMP at the Vienna BioCenter
The Research Institute of Molecular Pathology (IMP) in Vienna is a basic life science research institute largely sponsored by Boehringer Ingelheim. With over 220 scientists from 40 countries, the IMP is committed to scientific discovery of fundamental molecular and cellular mechanisms underlying complex biological phenomena. The IMP is part of the Vienna BioCenter, one of Europe’s most dynamic life science hubs with 2,800 people from over 80 countries in six research institutions, two universities, and 35 biotech companies. www.imp.ac.at, www.viennabiocenter.org