"Guardian of the Cell": structure and key function of giant protein explained
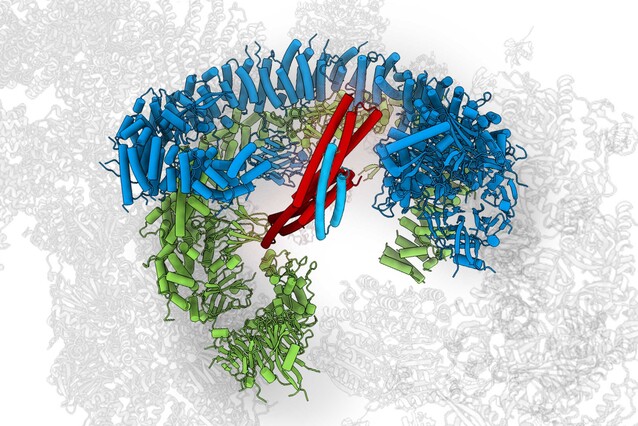
The enormous protein BIRC6 plays an important role in preventing programmed cell death – earning BIRC6 the nickname “Guardian of the Cell”. Scientists from the lab of Tim Clausen now showed that BIRC6’s structure holds the key for regulating cell death, but also for cell survival pathways. Their findings are published in the journal Science.
During an organism's healthy development but also during times of stress and pathology, surplus or damaged cells are eliminated by programmed cell death, also called apoptosis. When the molecular pathways that control apoptosis go haywire, diseases such as cancer can occur. In healthy systems, a class of proteins called Inhibitor of Apoptosis Proteins (IAPs) prevent this by blocking enzymes without which apoptosis cannot happen. BIRC6 is one of these IAPs, and so important for preventing self-destruction of the cell that it is nicknamed the “Guardian of the Cell”.
At a weight of one megadalton, the BIRC6 dimer is enormous - one of the larges known enzymes in human cells. So huge, in fact, that scientists were long unable to determine its structure. Traditionally, molecular structures were determined by X-ray crystallography, a technique for which the protein in question needs to be purified, turned into a crystal, and irradiated with high-energy beams. The resulting radiation patters would provide clues on the structure of the protein. However, this requires proteins to be small and robust –enormous BIRC6 was far off limits.

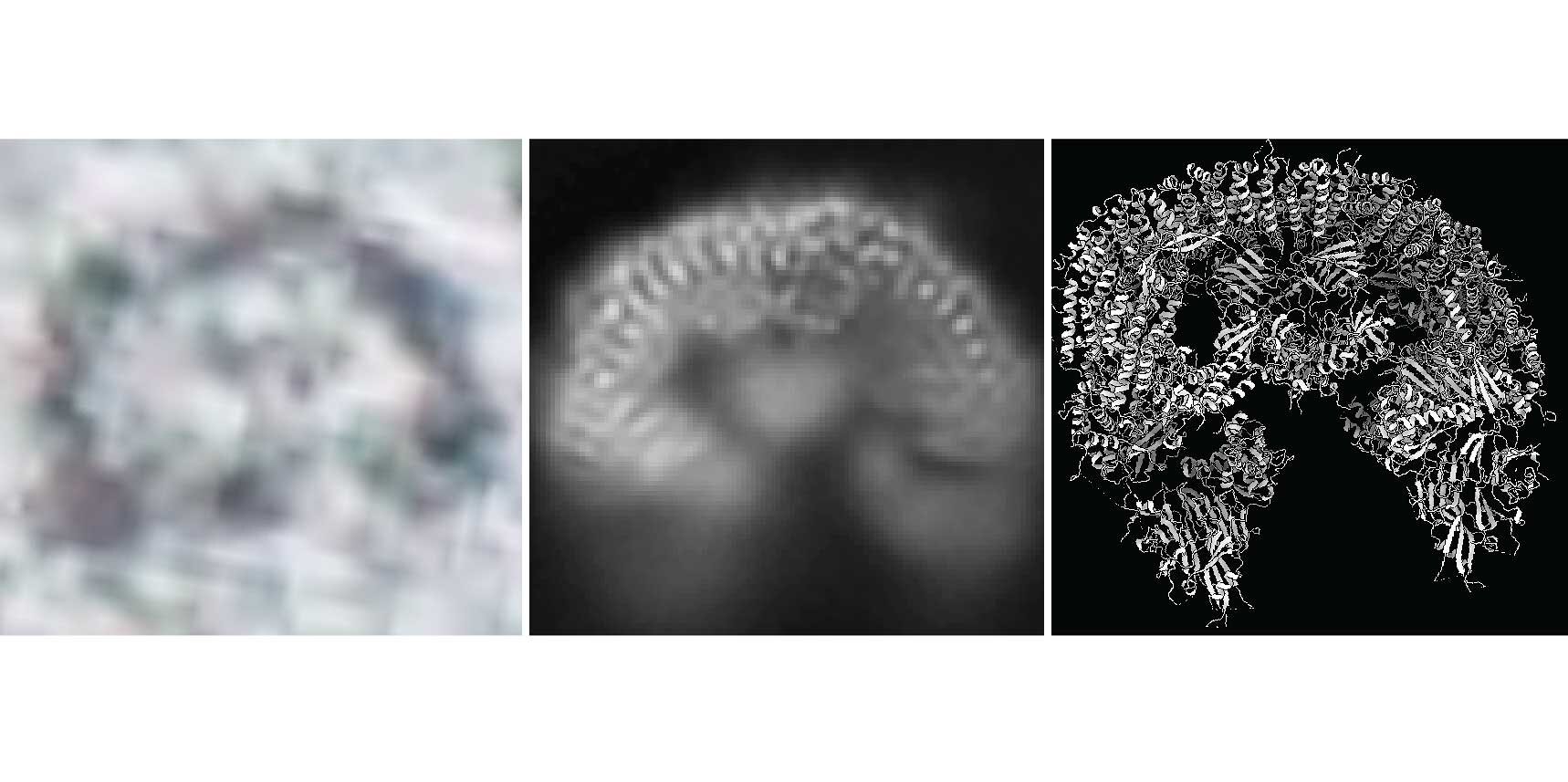
Scientists from the lab of Tim Clausen instead turned to a different technology, one that has recently pushed the boundaries of its resolution to new levels: cryo-electron microscopy. To study proteins with cryo-EM, there is no need to turn them into crystals, opening new opportunities to study the structure and function of proteins that had previously been inaccessible.
In spring 2022, a KRIOS G4 cryo-Electron Microscope was installed at the IMP. The state-of-the-art device provided the vital boost to the BIRC6 project: the scientists quickly accumulated structural data that allowed the detailed characterisation of the entire protein. While knowing the structure of BIRC6 marks a significant milestone in itself, the study now published in Science goes far beyond.
BIRC6 is the only essential IAP which blocks programmed cell death. It does this by binding enzymes required for apoptosis. In turn, BIRC6 is inhibited by SMAC, a small, apoptosis-inducing protein released from stressed mitochondria. SMAC frees apoptotic proteases from BIRC6 to initiate cell death, but how it can do this was not understood at the molecular level.
The scientists could show that BIRC6 has two curved branches with an active zone in the middle. SMAC can enter this middle like a key enters a lock, bind tightly and block access to the active zone, thereby preventing the reaction required for inhibiting apoptosis.
The large proteins that the Clausen lab studies are often involved in multiple molecular pathways and BIRC6 is no exception. BIRC6 also inhibits autophagy, the re-use of old or damaged cell components. “This is one of the really exciting aspects of this study,” says Tim Clausen. “Setting out from the structure of BIRC6, we could show exactly how it contributes to two fundamental mechanisms of cell biology, how it protects cells from premature apoptosis, and how the very strong SMAC binding co-regulates both cell death and cell survival pathways.”
The study demonstrates that the “cryo-EM revolution” has rung in a new era of structural biology: the structure and functions of very large proteins, which were previously out of reach, can now be studied in detail. It also creates the foundation for further investigations of additional pathways in which BIRC6 plays a role, as well as on the crosstalk between apoptosis and autophagy.
Future research on IAPs, however, is likely to build on medical potential: "Pinpointing the structure and function of BIRC6 creates the foundation we need to understand its roles in other pathways, in health and disease,” says Tim Clausen. “Dysregulated apoptosis can lead to diseases such as cancer and the role of IAPs such as BIRC6 for the inhibition of programmed cell death therefore makes them attractive drug targets.”
Original Publication
Julian F. Ehrmann, Daniel B. Grabarczyk, Maria Heinke, Luiza Deszcz, Robert Kurzbauer, Otto Hudecz, Alexandra Shulkina, Rebeca Gogova, Anton Meinhart, Gijs A. Versteeg, Tim Clausen (2023).
“Structural basis for regulation of apoptosis and autophagy by the BIRC6/SMAC complex” DOI: 10.1126/science.ade8873
Further Reading
The paper was submitted to Science back-to-back with the study "Structural basis for SMAC-mediated antagonism of caspase inhibition by the giant ubiquitin ligase BIRC6" by scientists from Paul Elliott’s lab of the University of Oxford, which was published in the same issue. DOI: 10.1126/science.ade8840