Transcriptional control of B cell development and immunity
B cell commitment and development
A fundamental question in hematopoiesis is how stem cells and early progenitors become committed to a single developmental pathway, and then differentiate into mature cell types of the selected lineage. The entry of lymphoid progenitors into the B cell lineage depends on several transcription factors, including E2A, EBF1, and Pax5. E2A and EBF1 function as B cell specification factors while our work has identified Pax5 as the critical B cell commitment factor that restricts the developmental potential of lymphoid progenitors to the B cell pathway. More recently, we have shown that the transcription factor Ikaros controls signaling downstream of the pre-B cell receptor by activating genes coding for signal transducers and repressing genes involved in the downregulation of pre-BCR signaling. Moreover, we have demonstrated an essential role for the transcription factor Bhlhe41 in controlling the development and self-renewal of innate-like B cells.
B cell immunity and plasma cell function
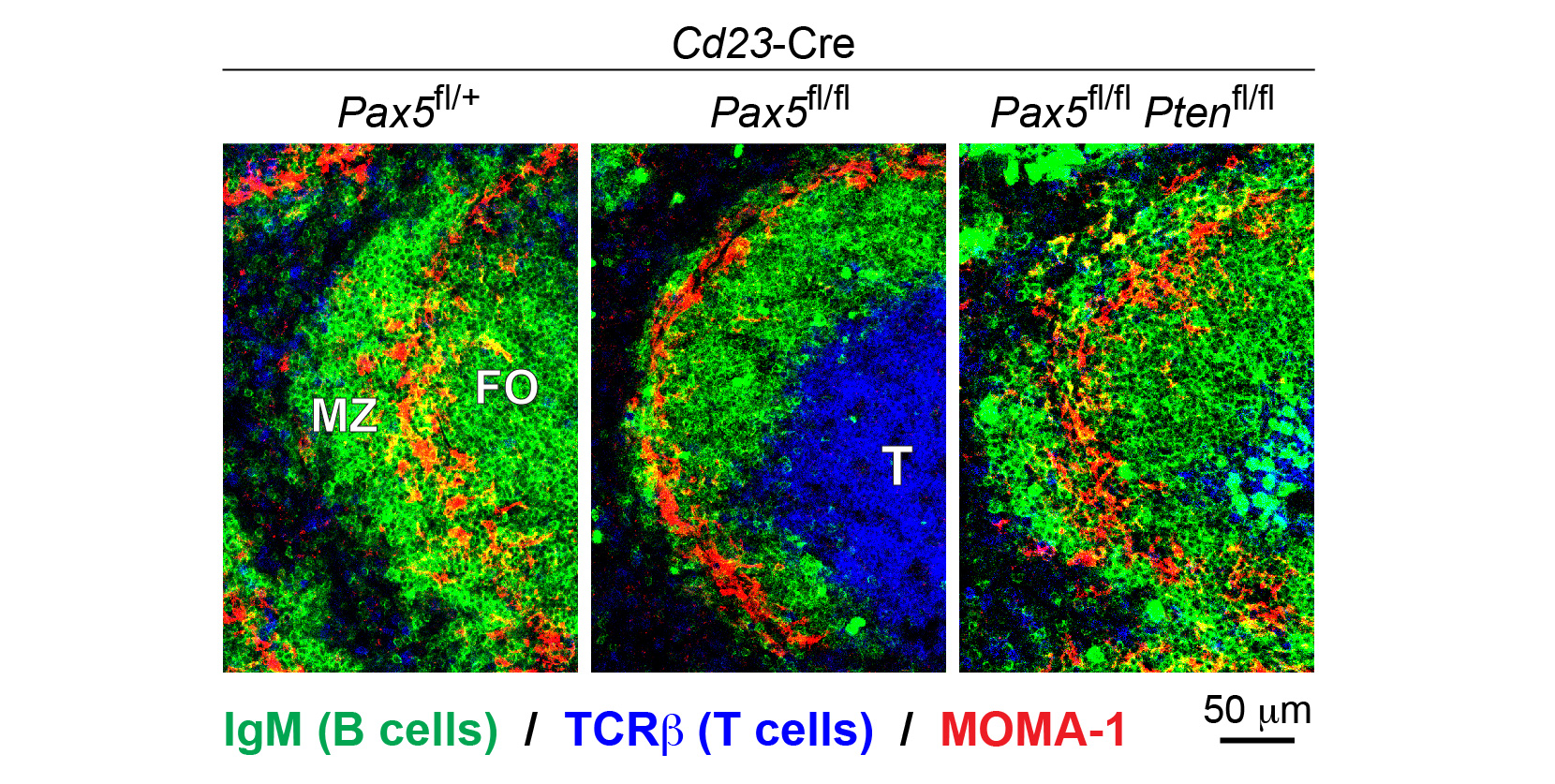
Different mature B cell types play an important role in mounting an efficient immune response to foreign pathogens. By conditionally inactivating Pax5 in mature B cells, our group recently discovered that Pax5 is essential for the generation and maintenance of all mature B cell types, as exemplified by the loss of marginal zone (MZ) B cells and a strong reduction of follicular (FO) B cells in the spleen (Figure 1). At the molecular level, Pax5 controls the PI3K-AKT signaling pathway downstream of the B cell antigen receptor (BCR) and Toll-like receptors (TLRs) by restraining the expression of PTEN, a negative regulator of the pathway. Notably, the combined loss of Pax5 and PTEN rescues the generation of MZ and FO B cells (Figure 1).
Upon encounter of foreign antigens, mature B cells undergo affinity maturation of their BCR and subsequently differentiate to plasma cells that provide acute and long-term protection against pathogens by generating and secreting high-affinity antibodies. Whereas the immune function of mature B cells strictly depends not only on Pax5, but also on E2A, EBF1 and Ikaros, the transcriptional regulator Blimp1 controls terminal differentiation to plasma cells. By analyzing the molecular mechanism of Blimp1 action, we could demonstrate that Blimp1 shuts down the B cell gene expression program by repressing B-cell-specific genes, while promoting plasma cell differentiation by activating genes involved in the unfolded protein response and in immunoglobulin secretion.
B cell leukemia and autoimmunity
PAX5 acts as a potent tumor suppressor gene in B cells, as heterozygous loss of PAX5 is observed in one third of all B-cell acute lymphoblastic leukemia (B-ALL) cases, while PAX5 translocations are also associated with B-ALL. By generating mouse models for the human PAX5-ETV6 and PAX5-JAK2 translocations, we could demonstrate that PAX5-ETV6 functions as a potent oncoprotein in combination with loss of the tumor suppressors CDKN2A,B. In contrast, the PAX5-JAK2 translocation acts as a dual-hit mutation by inactivating the PAX5 function of the translocated allele, while the PAX5-JAK2 fusion protein constitutively activates JAK-STAT signaling.
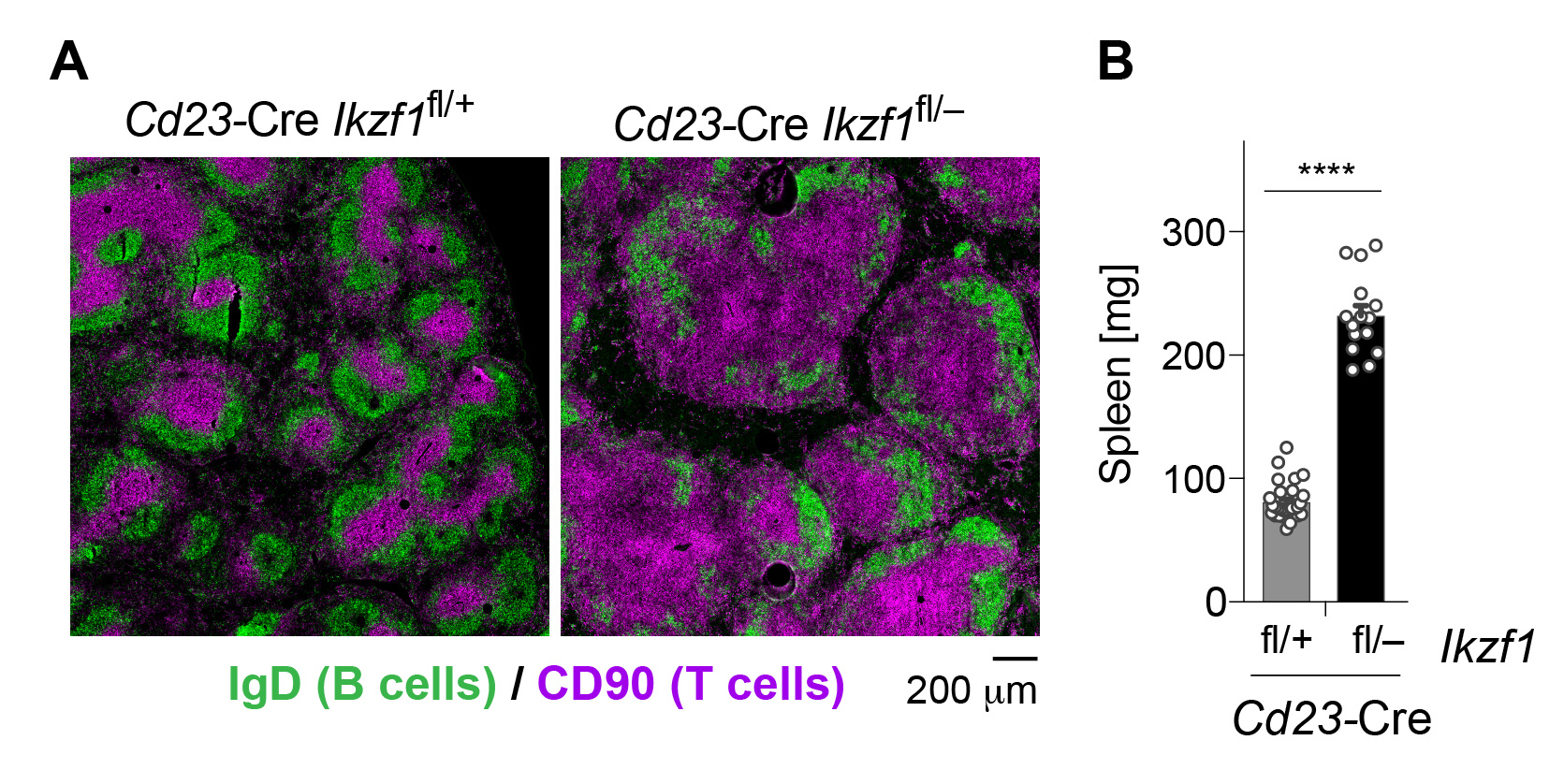
By conditionally inactivating Ikzf1 (Ikaros) in mature B cells, we identified a critical role for this transcription factor in the prevention of autoimmunity. The systemic inflammation observed upon Ikaros loss is manifested by a severely disorganized follicular structure (Figure 2A) and a strongly increased size (Figure 2B) of the spleen in Cd23-Cre Ikzf1fl/– mice. At the molecular level, Ikaros acts as a guardian preventing autoimmunity by promoting anergy induction in autoreactive B cells and by restraining innate TLR signaling in B cells. Heterozygous IKAROS mutations have subsequently been identified in patients with the autoimmune disease systemic lupus erythematosus.
Wapl repression by Pax5 promotes V-DJ recombination by inducing extended loop extrusion across the Igh locus
The development of B cells depends on the functional rearrangement of the immunoglobulin heavy-chain (Igh) and -light-chain (Igk) gene loci. Both antigen receptor loci are large in size (3 megabases), have a complex structure, and undergo reversible contraction in the Igh-rearranging pro-B cells or Igk-rearranging pre-B cells, as shown by our group. Locus contraction juxtaposes distantly located V genes of the large V gene cluster next to the DJ or J gene segments in the 3’ proximal domain, which facilitates the participation of all V genes in V-(D)J recombination, thus leading to a diverse antibody repertoire, providing humoral immunity to all possible pathogens.
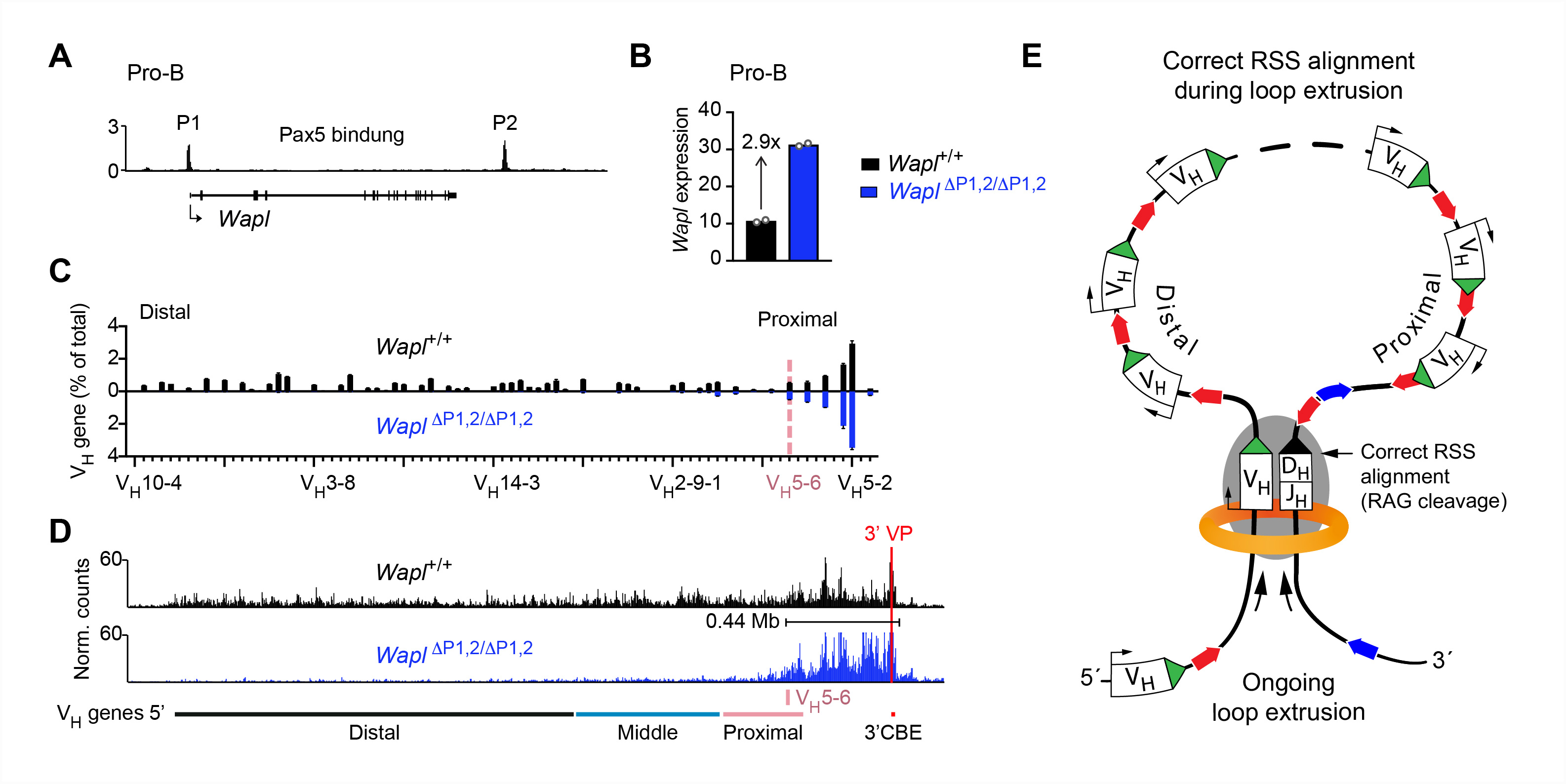
We previously identified an essential role of Pax5 in the control of Igh locus contraction. Elucidating the role of Pax5 in this process brought us into the field of chromosome biology, as the spatial regulation of V(D)J recombination is determined by the three-dimensional chromosomal organization consisting of compartments and topologically associated domains (TADs). TADs are formed by chromatin loop extrusion, which depends on the ring-shaped cohesin complex with its loop extrusion function. The cohesin-release factor Wapl instead restricts loop extension. We have recently discovered that locus contraction is caused by extended loop extrusion across the entire Igh locus (Figure 3E). Importantly, the expression of Wapl is repressed by Pax5 specifically in pro-B cells, which facilitates extended loop extrusion by increasing the residence time of cohesin on chromatin (Figure 3D). Pax5 mediates the transcriptional repression of Wapl through a single Pax5-binding site by recruiting the Polycomb repressive complex 2 to induce bivalent chromatin at the Wapl promoter (Figure 3A,B). Ongoing loop extrusion across the Igh locus at low Wapl expression facilitates the convergent alignment of individual VH genes with the DJH-rearranged gene segment located in the 3’ proximal, RAG endonuclease-containing recombination center, which leads to RAG-mediated cleavage (Figure 3E) and to the recombination of all VH genes in pro-B cells (Figure 3C).
Selected Publications
-
Fedl, AS., Tagoh, H., Gruenbacher, S., Sun, Q., Schenk, RL., Froussios, K., Jaritz, M., Busslinger, M., Schwickert, TA. (2024) Transcriptional function of E2A, Ebf1, Pax5, Ikaros and Aiolos analyzed by in vivo acute protein degradation in early B cell development. Nat Immunol. 25(9):1663-1677
-
Hill, L., Wutz, G., Jaritz, M., Tagoh, H., Calderón, L., Peters, JM., Goloborodko, A., Busslinger, M. (2023) Igh and Igk loci use different folding principles for V gene recombination due to distinct chromosomal architectures of pro-B and pre-B cells.
Nat Commun. 14(1):2316 -
Kaiser, FMP., Gruenbacher, S., Oyaga, MR., Nio, E., Jaritz, M., Sun, Q., van der Zwaag, W., Kreidl, E., Zopf, LM., Dalm, VASH., Pel, J., Gaiser, C., van der Vliet, R., Wahl, L., Rietman, A., Hill, L., Leca, I., Driessen, G., Laffeber, C., Brooks, A., Katsikis, PD., Lebbink, JHG., Tachibana, K., van der Burg, M., De Zeeuw, CI., Badura, A., Busslinger, M. (2022) Biallelic PAX5 mutations cause hypogammaglobulinemia, sensorimotor deficits, and autism spectrum disorder. J Exp Med. 219(9)
-
Hill, L., Ebert, A., Jaritz, M., Wutz, G., Nagasaka, K., Tagoh, H., Kostanova-Poliakova, D., Schindler, K., Sun, Q., Bönelt, P., Fischer, M., Peters, JM., Busslinger, M. (2020)
Wapl repression by Pax5 promotes V gene recombination by Igh loop extrusion. Nature 584:142-147. -
Schwickert, TA., Tagoh, H., Schindler, K., Fischer, M., Jaritz, M., Busslinger, M. (2019) Ikaros prevents autoimmunity by controlling anergy and Toll-like receptor signaling in B cells. Nat Immunol. 20(11):1517-1529