Internal and external factors that shape behaviour
From the earliest developmental timepoints, evolutionary-engrained patterns of neural wiring that form the basis for connectivity networks governing brain functions, are modulated by internal and external stimuli. Studying behaviour enables us to determine the resulting consequences by the evaluation of defined displays that determine survival and reproduction as key features of life on earth. These include aspects of emotional and social behaviours that have remained largely conserved throughout evolution, can be studied in animal models and mapped onto defined neural pathways in the brain.
Maternal immune activation during pregnancy
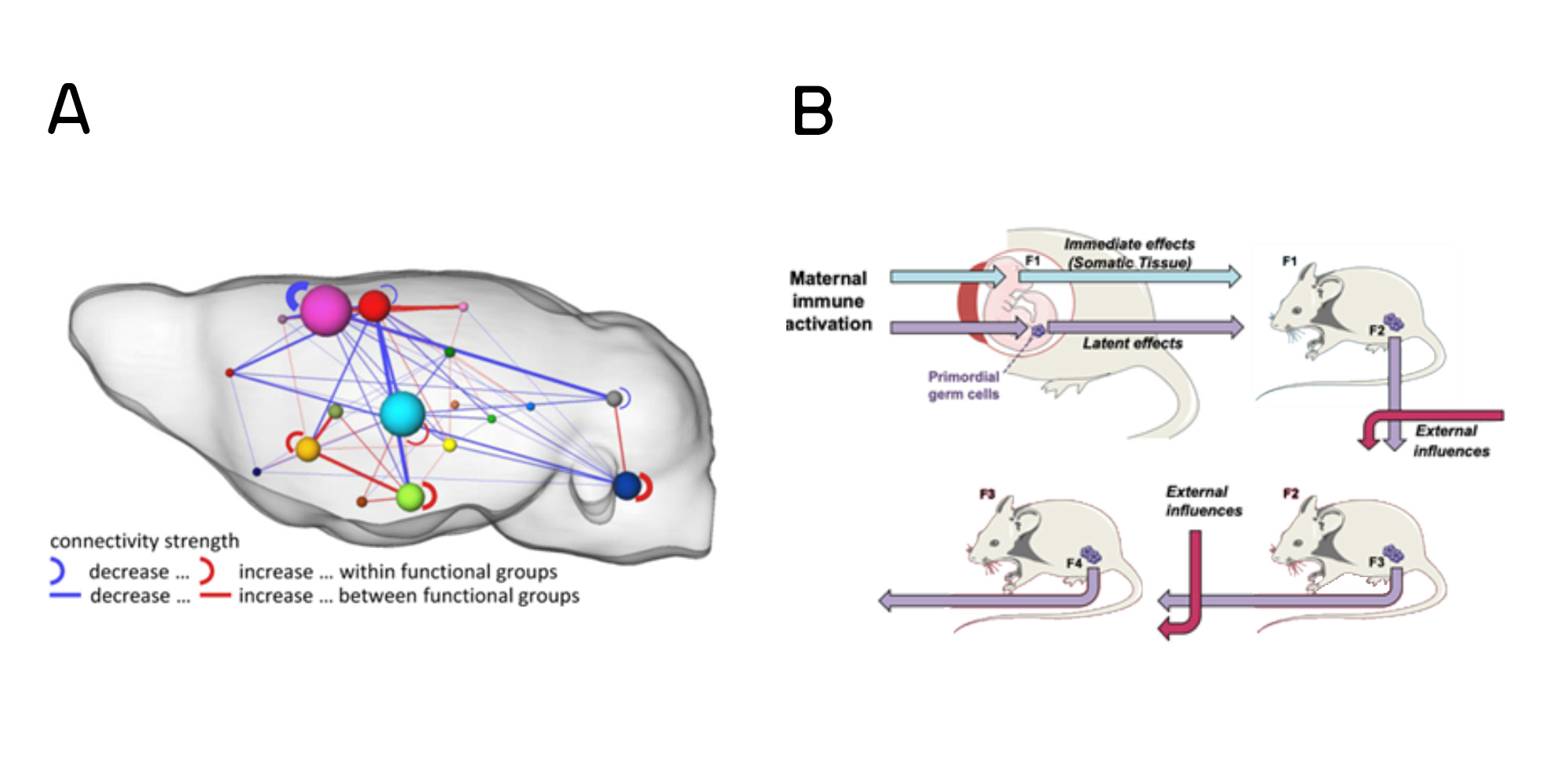
The prenatal environment plays a significant role in shaping neural development herby influencing the trajectory of behavioural patterns in the offspring. Maternal stress, nutrition, and exposure to infections during pregnancy modulate the prenatal environment and can impact the developing brain.
Maternal immune activation (MIA), mimicking viral infection during pregnancy is a risk factor for the development of psychopathologies later in life. In mice, MIA derails offspring brain development to induce long-lasting molecular-epigenetic, structural, and functional network connectivity modifications that underly behavioural output. We have found aberrant resting-state connectivity, smaller brain regions and less effective fibre structures as possible correlates of the behavioural phenotype in the MIA progeny. These data on dysfunctional network activity complements our earlier work on the cellular and molecular signature of MIA in the offspring brain. Importantly, we also found that the behavioural manifestations which include altered emotional behaviour were also passed on to the F2 generation without further immunogenic stimulation (Figure 1).
In addition to mood-related behavioural phenotypes, we also identified impaired maternal care behaviour in MIA mothers and their F1 daughters. Given the importance of maternal care for the integrity of offspring brain development, social, cognitive, and emotional behaviours later in life, the impact of MIA may be exacerbated by the compromise in maternal care.
Plasticity of the maternal brain
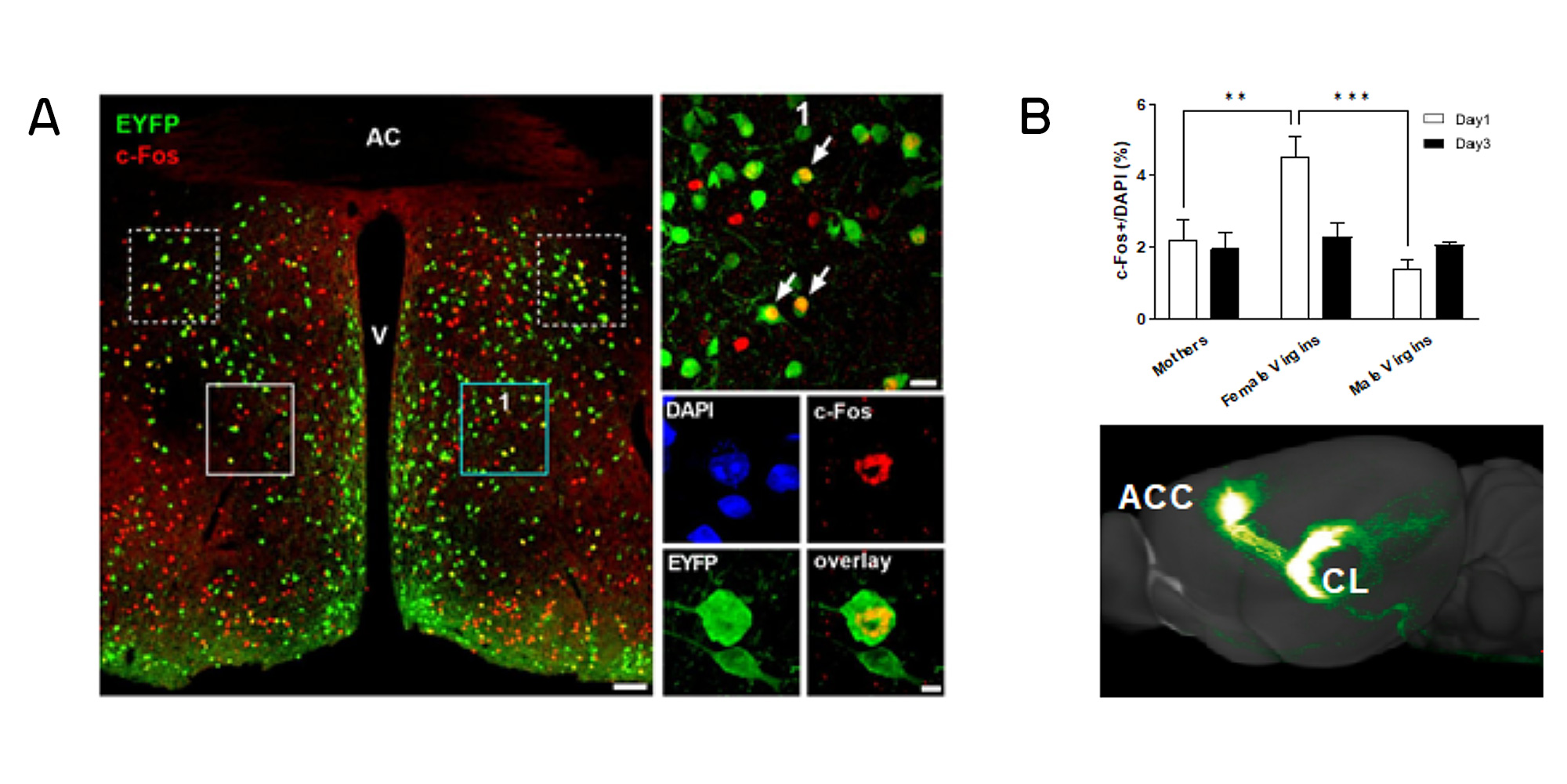
We were the first to show that MIA not only disrupts neural development in the foetus, but also critically impacts on the plastic rearrangements occurring during pregnancy in the female brain. We found that MIA compromised the integrity of the hypothalamic circuits modulating maternal care behaviour in the female brain (Figure 2). Consequently, MIA dams favoured non-pup directed exploratory behaviour at the expenses of pup caring behaviour.
It had long been considered that the hormonal influences during pregnancy are required to allow the immediate display of maternal care behaviour by postpartum females. We have recently discovered that nulliparous females can acquire parental care behaviour in the absence of pregnancy-related hormonal priming, by differential activation of a novel neural loop of direct connectivity between the prefrontal cortex and the thalamus (Figure 2). Male mice did not show recruitment of this circuit, suggesting the neural response to the same to the same reproductive cue to be dependent on sex and reproductive state of the individual.
Focus of ongoing research projects
- Impact of prenatal environment on brain development and behavioural outcomes: How does maternal nutrition during pregnancy impact on long-lasting brain and behaviour phenotypes in the offspring? What are the options for promoting resilience/ rescue pathological alterations in-utero and postnatally? How are transgenerational effects transmitted and is there an interaction between paternal and maternal contributions?
- Potential, limits and consequences of brain reproductive plasticity: Which are the biological basis and functional relevance of sex- and reproductive state-dependent rearrangements in the brain? Does enhanced malleability of the brain during changes in reproductive periods induce heightened susceptibility and what is the relevance for pathology?
Selected Publications
- An accessory prefrontal cortex-thalamus circuit sculpts maternal behavior in virgin female mice. Glat M, Gundacker A, Cuenca Rico L, Czuczu B, Ben-Simon Y, Harkany T, Pollak DD. EMBO J. 2022 Dec 15;41(24):e111648. doi: 10.15252/embj.2022111648.
- Gestational immune activation disrupts hypothalamic neurocircuits of maternal care behavior. Zambon A, Rico LC, Herman M, Gundacker A, Telalovic A, Hartenberger LM, Kuehn R, Romanov RA, Hussaini SA, Harkany T, Pollak DD. Mol Psychiatry. 2022 May 17:1-15. doi: 10.1038/s41380-022-01602-x.
- STAT3 in the dorsal raphe gates behavioural reactivity and regulates gene networks associated with psychopathology. Reisinger SN, Sideromenos S, Horvath O, Derdak S, Cicvaric A, Monje FJ, Bilban M, Häring M, Glat M, Pollak DD. Mol Psychiatry. 2021 Jul;26(7):2886-2899. doi: 10.1038/s41380-020-00904-2.
- Maternal immune activation during pregnancy impacts on brain structure and function in the adult offspring. Kreitz S, Zambon A, Ronovsky M, Budinsky L, Helbich TH, Sideromenos S, Ivan C, Konerth L, Wank I, Berger A, Pollak A, Hess A, Pollak DD. Brain Behav Immun. 2020 Jan;83:56-67. doi: 10.1016/j.bbi.2019.09.011.
- An animal model of a behavioral intervention for depression. Pollak DD, Monje FJ, Zuckerman L, Denny CA, Drew MR, Kandel ER. Neuron. 2008 Oct 9;60(1):149-61. doi: 10.1016/j.neuron.2008.07.041.