Wulf Haubensak is an Adjunct Investigator at the IMP and Professor at the Medical University of Vienna. You can read more about his work at his lab's website:
https://hirnforschung.meduniwien.ac.at/en/our-divisions/division-of-neuronal-cell-biology/
Affective processing
Individuals strive to avoid danger while pursuing opportunities, whether in the wild or in complex societies. These behaviors are guided by affective networks in the brain that detect threats and rewards, motivating appropriate responses.
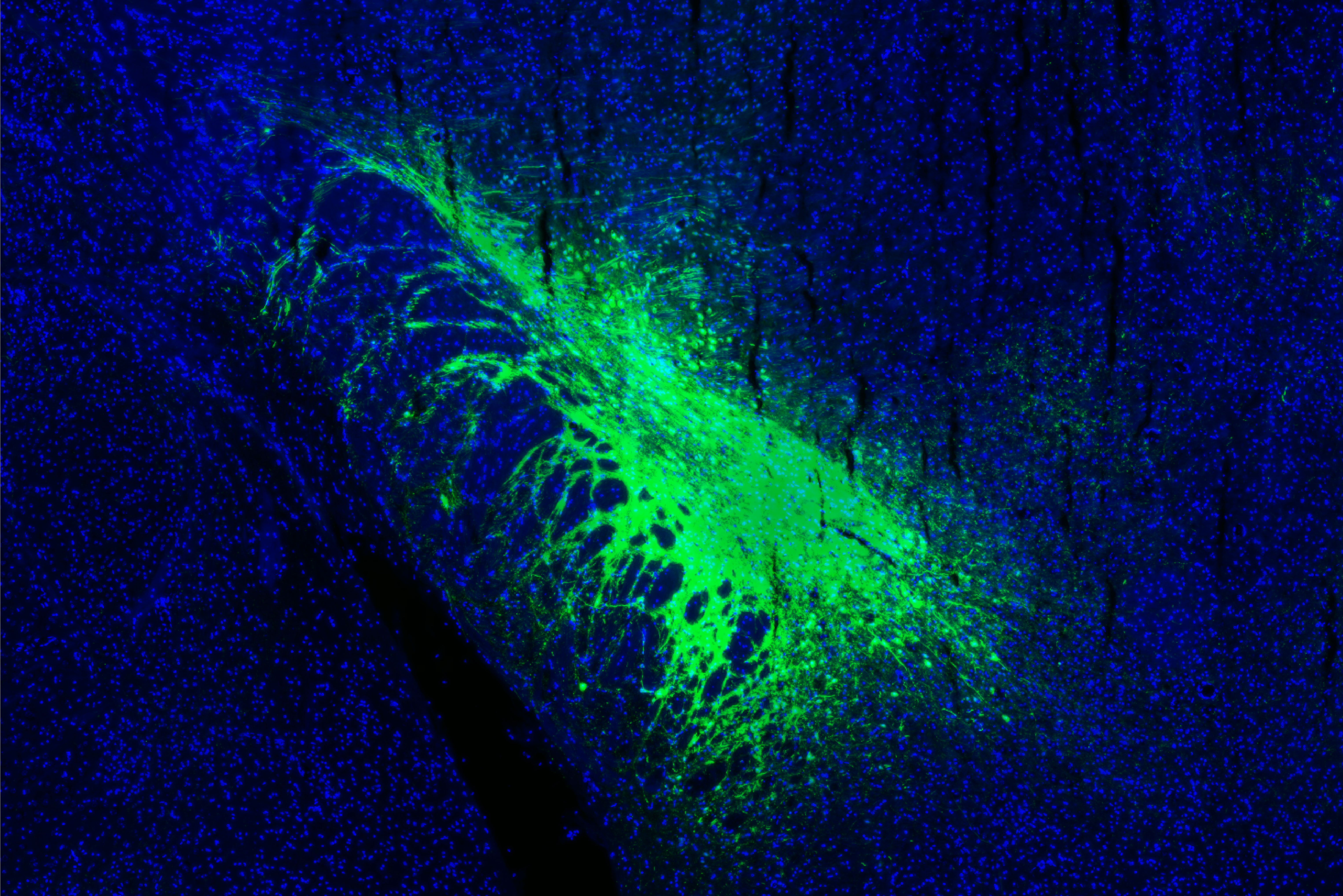
In a circuit neuroscience initiative, we deconstruct how the brain processes affective stimulus and transforms them into behavior at the neuronal network level. Our research combines techniques like neuronal tracing, genetics, and optogenetic manipulation to map brain circuits for affective processing. We integrate these methods with electrophysiology, Ca2+ imaging, and preclinical fMRI to explore how these circuits encode affective states, and how genes remodel circuit activity, emotional states, and behavioral responses.
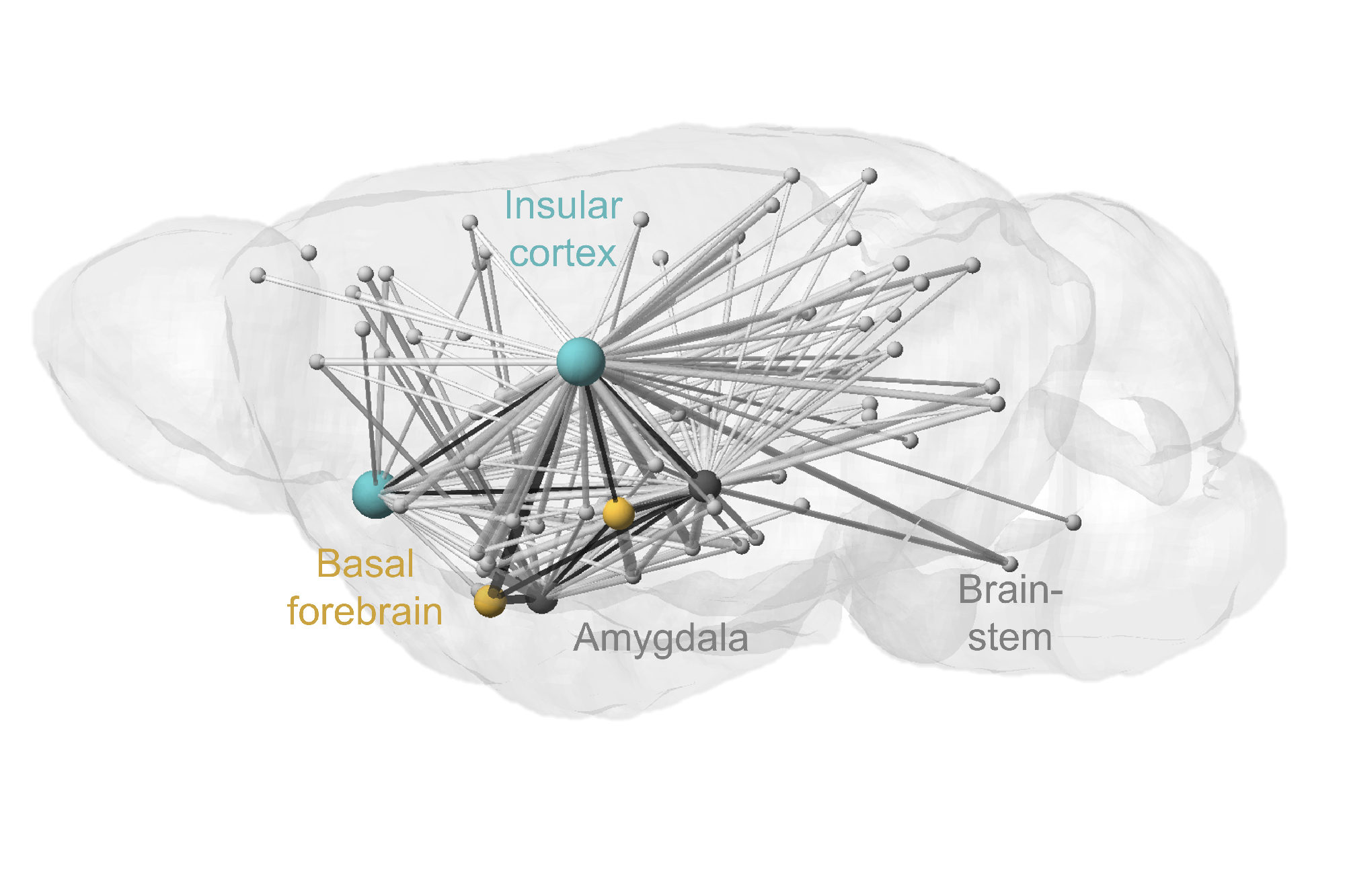
We have identified a cortico-limbic circuit module between the amygdala, brainstem, and insular cortex. This module serves as a model to study two crucial steps in this process (Grössl et al., 2018, Kargl et al., 2020): (1) How does the brain builds affective models and integrates interoceptive intuition (gut feelings) to assign an affective value—salience (importance) and valence (good or bad)—to environmental stimuli (Sladky et al., 2024)? and (2) How are these affective responses regulated in terms of space (object navigation) and time (impulse control) (Piszczek et al., 2022)?
Behavioural diversity
Individual brains interpret and respond to the world in unique ways. Some are more impulsive or dominant, while others might be more anxious. What factors contribute to this diversity? To a large degree, neuronal circuitry can be genetically programmed to influence specific behavioural biases manifesting as either, a behavioural trait or a psychiatric disease such as stress-related disorders. We hypothesize that most of the genetic variance accumulates along specific sites in the neuronal networks, biasing local computations, which underlie transitions between behavioural phenotypes and/or traits (Piszczek et al., 2022, Ganglberger et al., 2018).
In a neurogenetic initiative, we are currently implementing integrated workflows that connect circuit neuroscience with neurogenetic data (Pfaff et al., 2019), comparative neuroscience, and ecologically-relevant habitats. Furthermore, we are combining multimodal brain data across species (Ganglberger et al., 2024, Ganglberger et al., 2020), and across evolutionary phylogenies (Kaczanowska et al., 2022). This multimodal comparative approach allows us to investigate how sets of genes might bias circuit activity and behavioural responses, within populations, between species, and across evolutionary timescales. Ultimately, this holistic strategy aims to enhance our understanding of the factors that constrain and promote the relation between genetic variance, circuit computation, and affective traits – processes that drive behavioural diversity in health and psychiatric conditions, while also shedding light on the evolutionary trajectories of the mammalian brain (Piszczek et al., 2024).
Selected Publications
- J Kaczanowska, F Ganglberger, O Chernomor, D Kargl, B Galik, A Hess, Y Moodley, A von Haeseler, K Bühler, W Haubensak (2022). Molecular archaeology of human cognitive traits. Cell Reports 40 (9) https://www.cell.com/cell-reports/fulltext/S2211-1247(22)01107-X
- L Piszczek, A Constantinescu, D Kargl, J Lazovic, A Pekcec, J R Nicholson, W Haubensak (2022) Dissociation of impulsive traits by subthalamic metabotropic glutamate receptor 4. Elife 11, e62123. https://elifesciences.org/articles/62123
- J Griessner, M Pasieka, V Böhm, F Grössl, J Kaczanowska, P Pliota, D Kargl, B Werner, N Kaouane, S Strobelt, S Kreitz, A Hess, W Haubensak (2021). Central amygdala circuit dynamics underlying the benzodiazepine anxiolytic effect Molecular psychiatry 26 (2), 534-544. https://www.nature.com/articles/s41380-018-0310-3
- D Kargl, J Kaczanowska, S Ulonska, F Groessl, LPiszczek, J Lazovic, K Buehler, WHaubensak (2020) The amygdala instructs insular feedback for affective learning. Elife 9, e60336. https://elifesciences.org/articles/60336
- F Ganglberger, D Kargl, M Töpfer, J Hernandez-Lallement, N Lawless, F Fernandez-Albert, W Haubensak, K Bühler (2024). BrainTACO: an explorable multi-scale multi-modal brain transcriptomic and connectivity data resource. Communications Biology 7 (1), 730. https://www.nature.com/articles/s42003-024-06355-7